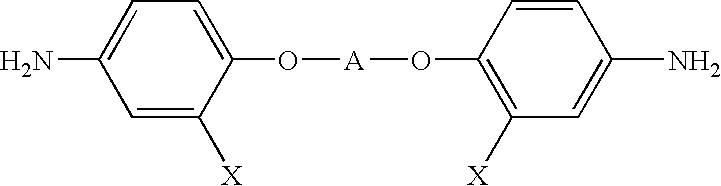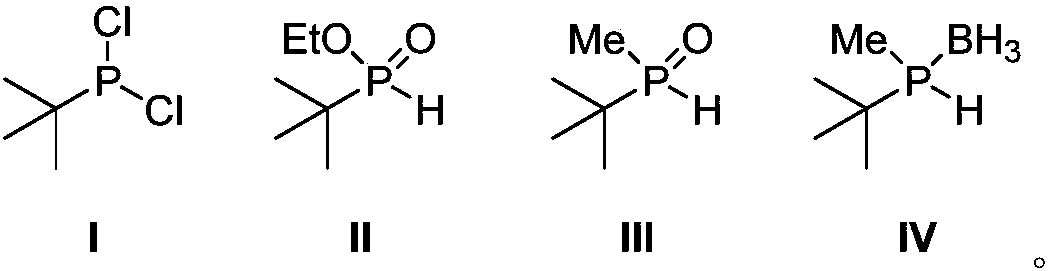Patents
Literature
72 results about "Tert-Butyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tert-Butyl chloride is the organochloride with the formula (CH₃)₃CCl. It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol. It is produced industrially as a precursor to other organic compounds.
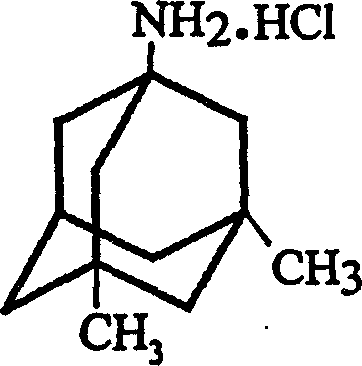
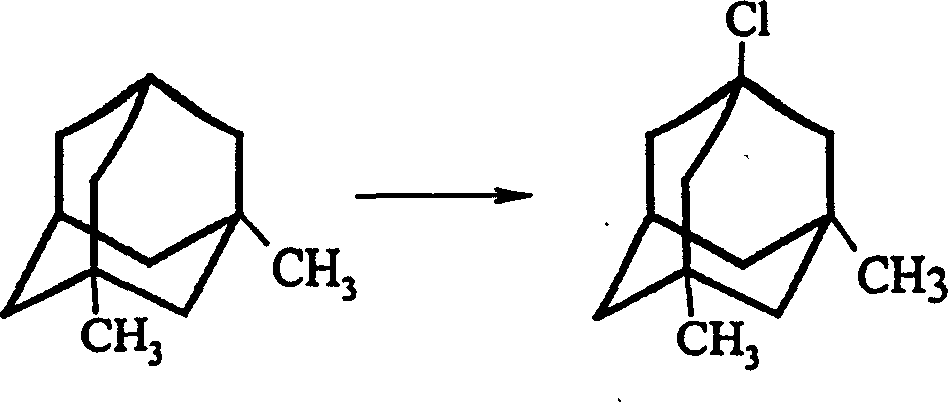
Method for preparing memantine hydrochloride
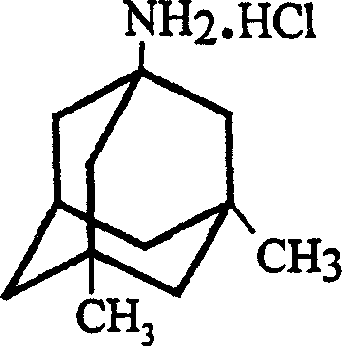
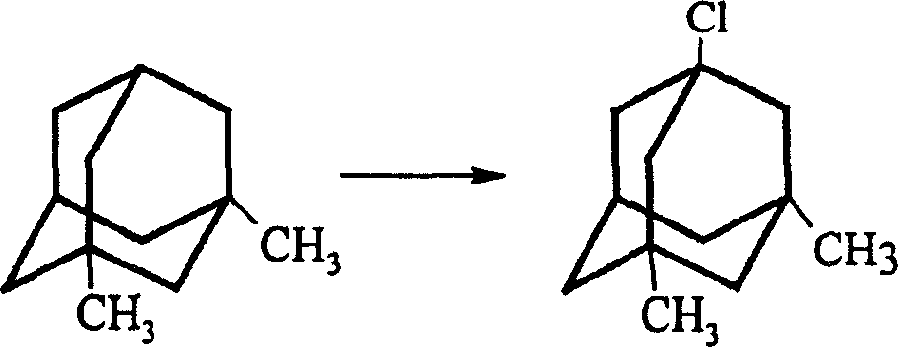
InactiveCN1488622AGood crystal formHigh purityNervous disorderPreparation by rearrangement reactionsMemantine HydrochlorideTert-Butyl chloride
The invention is a manufacturing method of diamante amine hydrochlorate. The invention adopts 1, 3-dimethyl adamantine to reacts with tert-butylchlorine and gets 1-chlorine-3, 5-dimethyl adamantine; then reacts with acetamide directly, the educt from water reacts with sodium hydroxide in ethanediol or glycerine solvent, extracts by acetic ester, condenses, and blow in dry hydrochloride gas and gets the coarse product. There gets the pure product through alcohol-acetic ester recrystallization.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Method for preparing butenafine hydrochloride
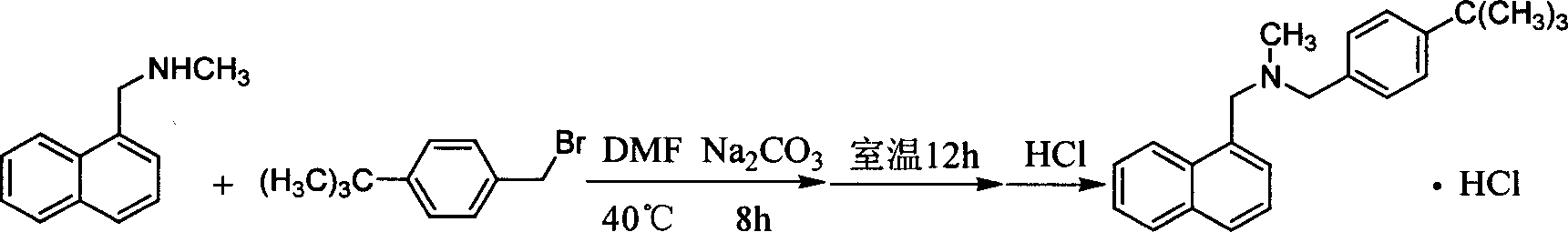
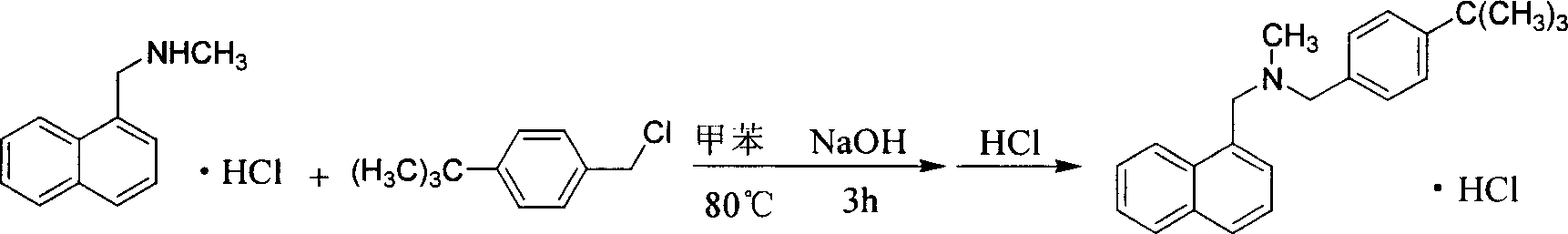
InactiveCN101077858AReduce pollutionSimple processOrganic active ingredientsAntimycoticsOrganic solventTert-Butyl chloride
The present invention provides process of preparing Butenafine hydrochloride. Under the catalysis of phase transfer catalyst and in water as the reaction solvent, p-tert-butyl benzyl chloride and N-methyl-1-naphthyl methylamine react to produce Butenafine hydrochloride. The preparation process is simple, uses no toxic organic solvent, and has less environmental pollution, lowered cost, raised yield and shortened production period.
Owner:凌沛学
Novel technology for synthesizing bactericide tebuconazole without solvent
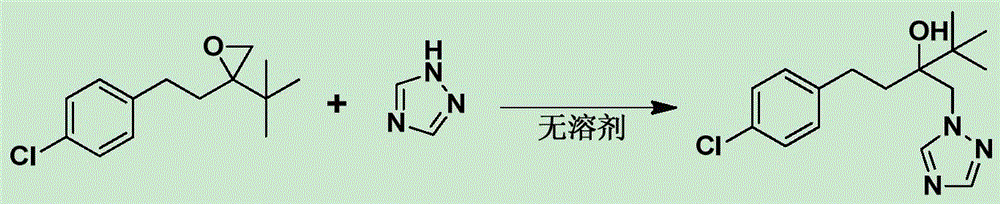
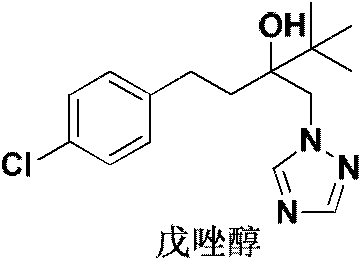
InactiveCN106588791ASimple and fast operationIncrease profitOrganic chemistryEthylene oxideSolvent free
The invention discloses a novel technology for preparing a bactericide tebuconazole through a solvent-free method. The technology comprises the steps that 2-tertiary butyl-2-(4-chlorphenyl ethyl)ethylene oxide and 1,2,4-triazole are taken as raw materials and react under the catalytic action of an alkaline catalyst and a phase transfer catalyst, and aftertreatment is conduced on a reaction product to obtain the product tebuconazole. According to the technology, no solvent is used in the synthesizing reaction process, and therefore the solvent cost input is reduced; in addition, due to the fact that the reaction concentration is high, the reaction speed is greatly increased, the product yield is significantly increased, a separation method is easy and convenient to operate, and industrialized production is easy.
Owner:YANCHENEG HUIHUANG CHEM
Method for preparing 3,3-dimethyl butyraldehyde
InactiveCN104130115AReduce manufacturing costShort production processOrganic compound preparationCarboxylic acid esters preparationDistillationTert-Butyl chloride
The invention relates to a method for preparing 3,3-dimethyl butyraldehyde. The method comprises the following steps: (1) vacuumizing a closed reaction kettle, adding a dichloromethane solvent, dripping vinyl acetate, introducing a catalyst after dripping is ended, and introducing hydrogen chloride gas, so that the pressure reaches 0-1MPa, slowly dripping tert butyl chloride for reacting, removing excessive hydrogen chloride gas after the reaction is ended to obtain a distillation product, raising the temperature and distilling to obtain 1-chloro-3,3-dimethyl butyl acetate; and (2) adding the 1-chloro-3,3-dimethyl butyl acetate and water into the reaction kettle, controlling the temperature to be 120 DEG C, performing hydrolysis disproportionation on the 1-chloro-3,3-dimethyl butyl acetate to obtain a hydrolysis disproportionation reaction mixture containing 3,3-dimethyl butyraldehyde, and distilling and purifying to obtain the 3,3-dimethyl butyraldehyde. According to the method disclosed by the invention, the production cost is low, the production process is shortened, the reaction conditions are mild, the production safety is high, and the preparation method is simple in preparation process, easy to control, high in yield and safe.
Owner:SHANDONG BENYUE BIOTECH
Preparation method of poly alpha-olefin base oil with high viscosity index
InactiveCN103305263AEasy to makeImmobilized reaction efficiency is highLiquid hydrocarbon mixtures productionHydrogen pressureDistillation
The invention discloses a preparation method of poly alpha-olefin (PAO) base oil with high viscosity index. Suitably, the method comprises the steps of: with ethylene and isobutene and other alpha-olefin of C6-C12 as raw materials and under the conditions of temperature of 20 DEG C-60 DEG C and partial hydrogen pressure of 0.3-0.5, loading a copper (II)-organic aluminum immobilized catalyst with gama-Al2O3, catalyzing ethylene / isobutene / alpha-olefin of C6-C12 for an oligomerization reaction for 2-4 hours by taking tert-butyl chloride as a catalyst promoter, and carrying out washing in water, drying, atmospheric distillation and reduced-pressure distillation on the product to obtain an olefin oligomer; and then under the conditions of temperature of 200 DEG C-280 DEG C, pressure of 2.5-5.0MPa, idle speed of 0.4-1.0h<-1>, and hydrogen-to-oil of 500:1, hydrogenating the oligomer through an Mo-Ni / gama-Al2O3 catalyst to obtain PAO (poly alpha olefin) base oil. According to the method, the yield of the PAO base oil is large, the kinematic viscosity of the oligomer is 27.8-42.4.0mm<2> / s at 100 DEG C, the viscosity index is 147-171, the pour point is minus 37 DEG C-minus 47 DEG C, and the bromine value is less than 0.050 bromine g / 100gPAO.
Owner:上海泰强粘合剂有限公司 +1
Method for the synthesis of low cost initiators for telechelic polyisobutylenes
Owner:THE UNIVERSITY OF AKRON
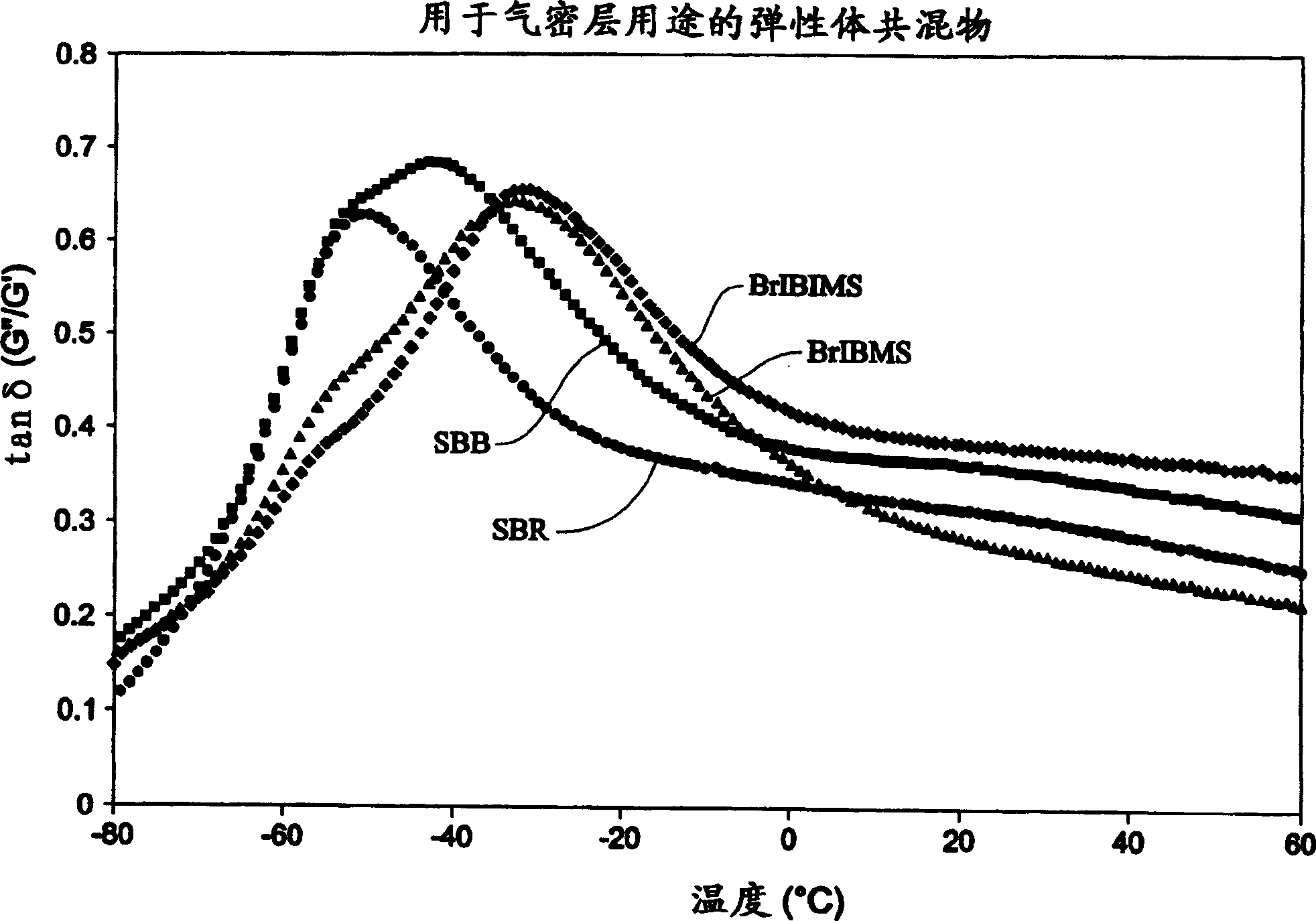
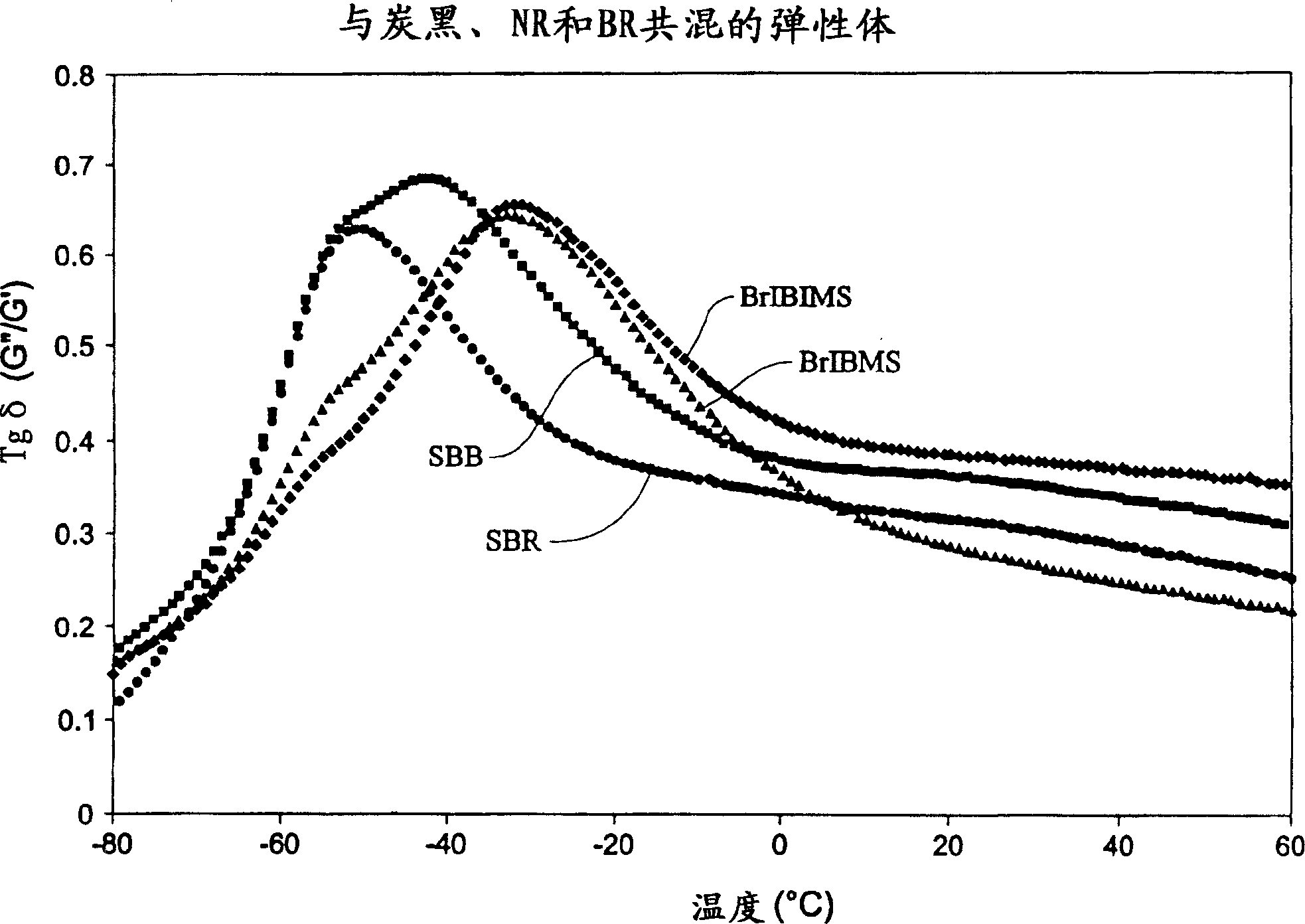
Elastomeric compositions
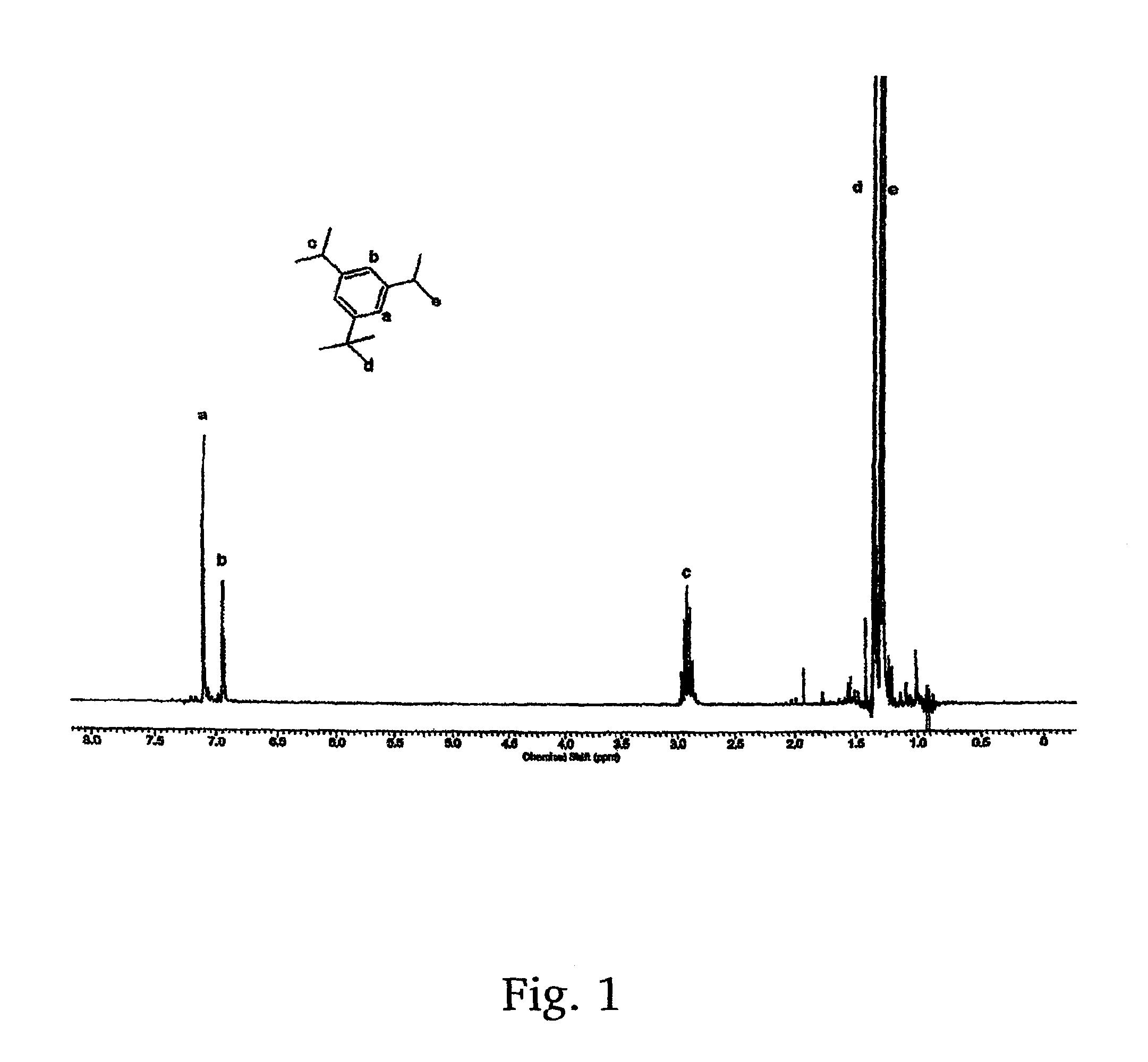
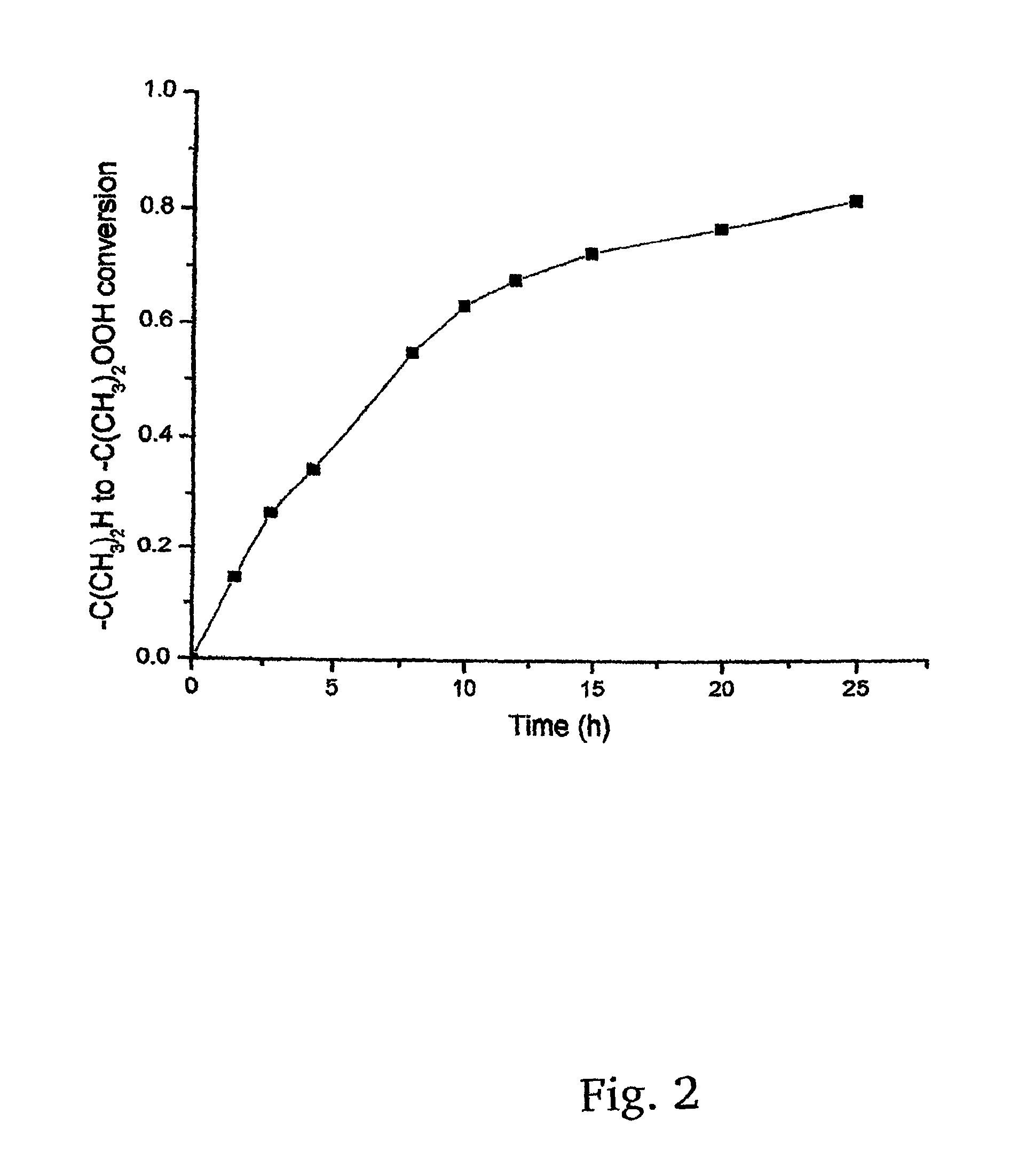
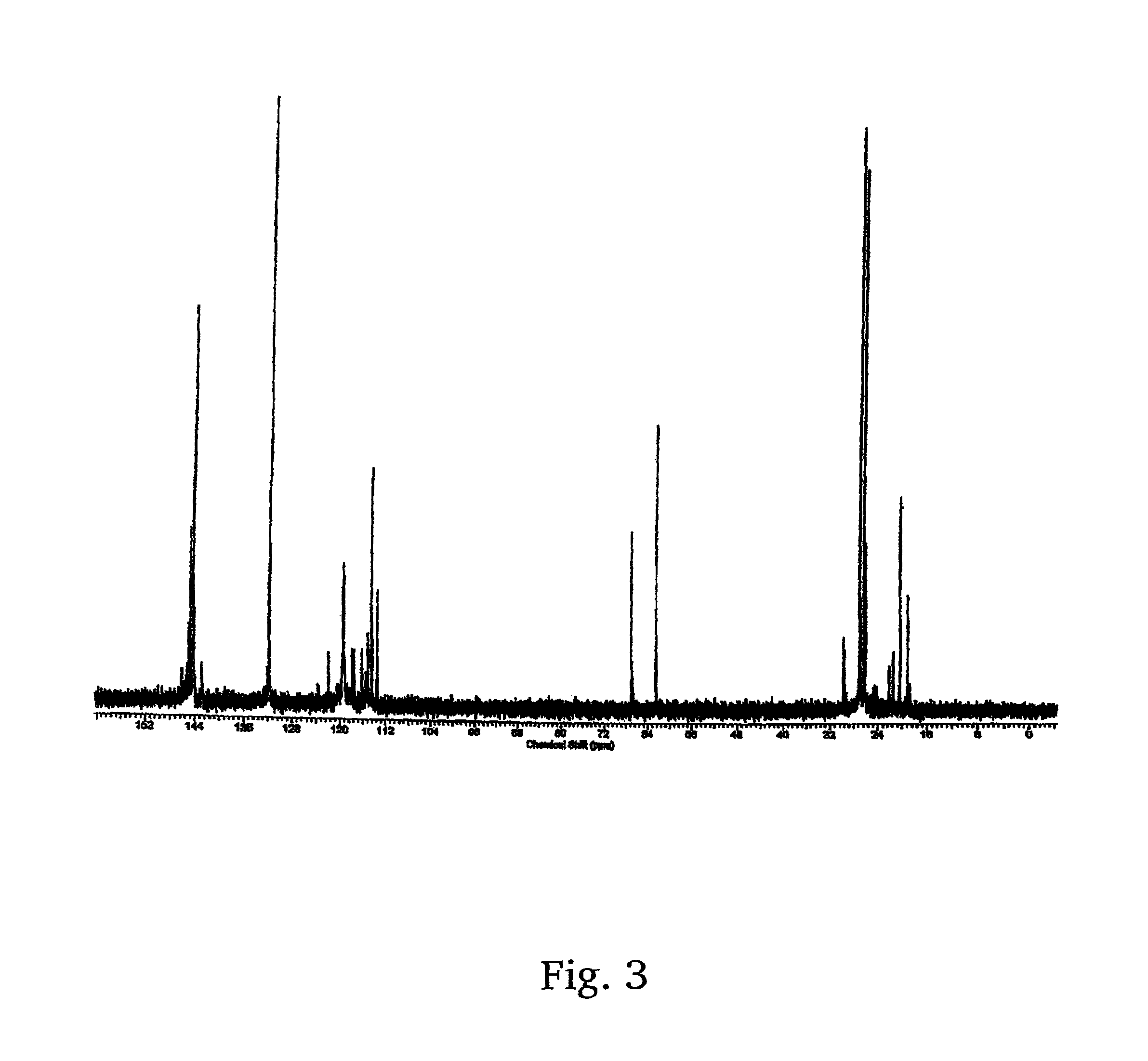
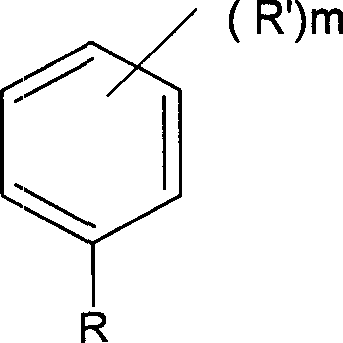
The present invention includes compositions suitable for use in tire treads or sidewalls and other articles requiring abrasion resistance and flexibility. The present invention includes tire treads or sidewalls prepared by combining: a filler; a sulfur-containing vulcanization system; optionally at least one second rubber; and at least one compound made of C 4 -C 8 Isoolefin derived units, C 4 -C 14 A halogenated terpolymer formed from polyolefin-derived units and p-alkylstyrene-derived units. Suitable examples of fillers include carbon black, silica and combinations thereof. The present invention also includes a method of producing an elastomeric terpolymer composition comprising combining C in a diluent in the presence of a Lewis acid and at least one initiator 4 -C 8 Isoolefin Monomer, C 4 -C 14 Polyolefin monomers, and p-alkylstyrene monomers to produce terpolymers. Examples of suitable initiators include cumyl compounds and / or halogenated organic compounds, especially secondary or tertiary halogenated compounds, for example, tert-butyl chloride, 2-acetyl-2-phenylpropane (cumyl acetate), 2- Methoxy-2-phenylpropane (cumyl methyl-ether), 1,4-bis(2-methoxy-2-propyl)benzene (bis(cumyl methyl ether)); cumyl halogenated compounds, especially chlorides, such as 2-chloro-2-phenylpropane, cumyl chloride ((1-chloro-1-methylethyl)benzene), 1,4-bis(2-chloro-2- Propyl)benzene (bis(cumyl chloride)) and 1,3,5-tris(2-chloro-2-propyl)benzene (tris(cumyl chloride)); aliphatic halides, especially chlorides, For example, 2-chloro-2,4,4-trimethylpentane (TMPCl) and 2-bromo-2,4,4-trimethylpentane (TMPBr).
Owner:EXXONMOBIL CHEM PAT INC
Manufacturing method of alkyl benzene solvent and its application
InactiveCN1824632AHigh selectivityHigh reaction yieldCopper organic compoundsHydrocarbonsReaction temperatureButyl chloride
The present invention relates to a preparation method of alkyl benzene solvent. It is made up by adopting tert-butyl chloride or tert.-butyl alcohol and dimethylbenzene or ethylbenzene through a certain reaction process. Its reaction temperature is 0-50 deg.C, reaction pressure is normal pressure, the mole ratio of tert.-butyl chloride or tert.-butyl alcohol and dimethylbenzene or ethylbenzene is 1.5-1-1:1.5, the catalyst dose is 0.5-20% of solvent weight. The catalyst is an ion liquid catalyst or one of anhydrous aluminium trichloride, anhydrous iron trichloride, sulfuric acid and hydrofluoric acid. The invented alkyl benzene solvent purity is 98%-100%, boiling range is 200-215 deg.C and relative density is 0.86-0.88. It can be used for synthesis of phthalocyanine copper.
Owner:彭光辉
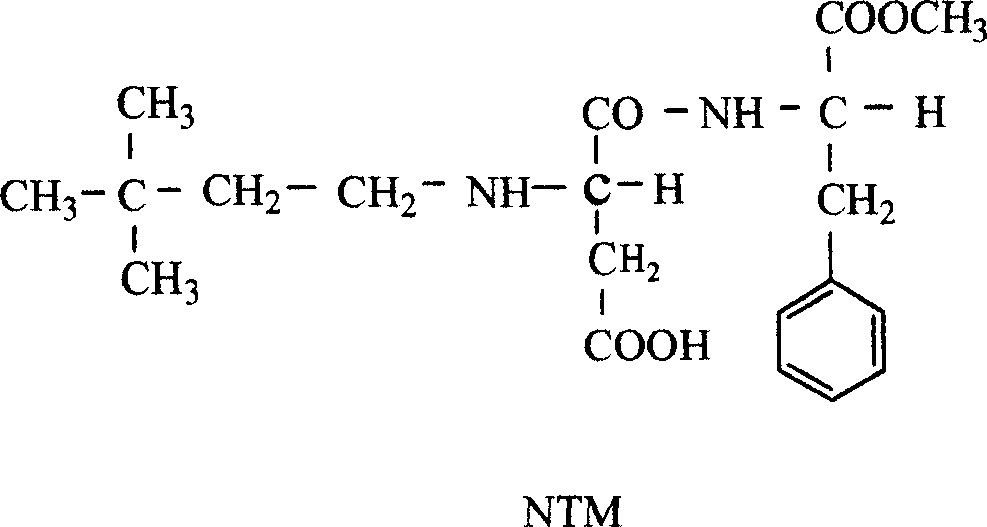
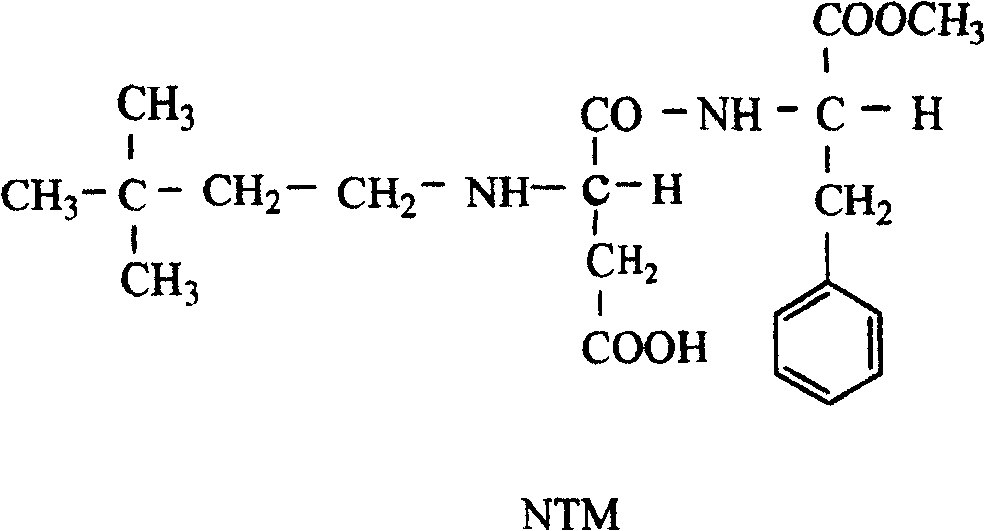
Synthetic methods for nutame
InactiveCN1966518ASimple processSimple and fast operationDipeptidesFood preparationSynthesis methodsTert-Butyl chloride
This invention discloses a synthesis method for Neotame. The said method includes the following steps: (1) carrying out a reaction of tert-butanol with hydrochloric acid to obtain tert-butyl chloride; (2) adding catalyst in tert-butyl chloride, and charging ethylene gas and reacting to obtain 3, 3-dimethyl-1-chlorobutane; (3) oxidating 3, 3-dimethyl-1-chlorobutane to obtain 3, 3-dimethyl butyraldehyde, and purifying; and (4) carrying out a reaction of 3, 3-dimethyl butyraldehyde with aspartame to form imine, and catalytic-hydrogenating to obtain Neotame. The invention has the advantages of simple process, easy operation, low cost, and high purity of the product.
Owner:JINAN UNIVERSITY
Method for preparing p-tert-butylbenzylamine
InactiveCN101704755AAvoid defectsOrganic compound preparationAmino compound preparationChemical synthesisQuaternary ammonium cation
The invention belongs to the technical field of compound preparation and discloses a chemical synthetic method for preparing p-tert-butylbenzylamine. The chemical synthetic method is characterized by comprising the following steps: firstly, reacting p-tert-butylbenz with urotropine to prepare quaternary ammonium salt, carrying out prehydrolysis on the quaternary ammonium salt by hydrochloric acid and alcohol solution, filtering, evaporating a solvent to obtain p-tert-butylbenzylamine hydrochloride solid; using the p-tert-butylbenzylamine hydrochloride solid as a raw material, using hydrochloric acid and water to carry out secondary hydrolysis on the p-tert-butylbenzylamine hydrochloride solid, reducing pressure, evaporating the solution to be dried, obtaining faint yellow solid matter, and using 40 percent sodium hydroxide solution to adjust pH of the faint yellow solid matter to be 8 to 12, thus obtaining the p-tert-butylbenzylamine. The chemical synthetic method can effectively remove a large amount of impurities in the p-tert-butylbenzylamine prepared by the existing method and obtain the p-tert-butylbenzylamine with high purity.
Owner:HUAZHONG AGRI UNIV
Preparation method of industrial musk tonalide
InactiveCN102050715AExtended reaction timeHigh selectivityCarbonyl compound preparation by condensationP-isopropyltolueneDistillation
The invention relates to a preparation method of industrial musk tonalide, which comprises the following steps: carrying out Friedel-Crafts alkylation by using p-isopropyl toluene and 2,3-dimethyl-1-butene as raw materials and using tertiary butyl chloride as a hydrogen absorption agent to synthesize an intermediate 1,1,3,4,4,6-hexamethyl tetrahydronaphthalene; and in a dichloromethane solvent, carrying out Friedel-Crafts alkylation on the intermediate used as the raw material and acetyl chloride, thereby obtaining the product musk tonalide (7-acetyl-1,1,3,4,4,6-hexamethyl tetrahydronaphthalene, AHMT). Compared with the existing method and technology, the method provided by the invention is simple to operate, has the characteristics of high reaction speed, high yield and the like, and realizes industrial production; and the product can be simply purified by thermally dissolving out pigments by using anhydrous alcohol without distillation or rectification.
Owner:HENAN UNIV OF SCI & TECH
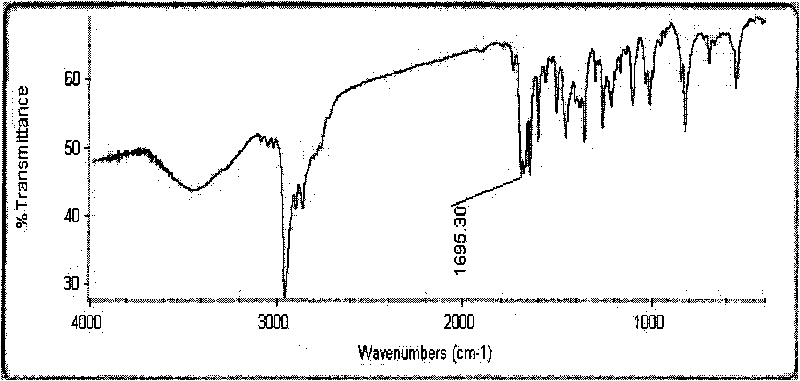
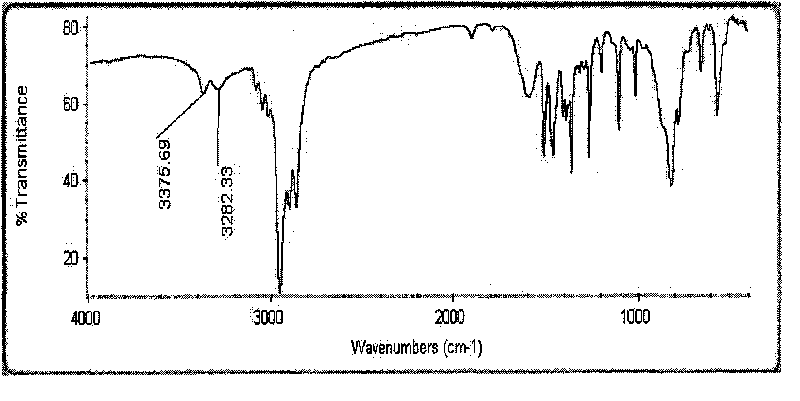
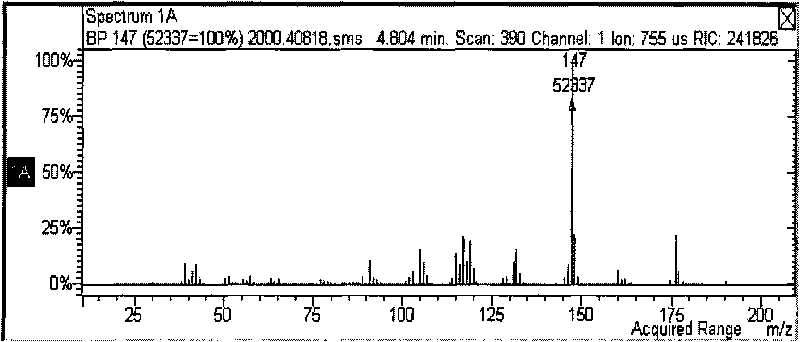
Method for synthesizing 1-cyclopropyl-naphthyl by virtue of 1-vinyl-naphthyl
InactiveCN103664471ALower synthesis costMild reaction conditionsHydrocarbon by hydrocarbon and non-hydrocarbon condensationOrganic synthesisGrignard reagent
The invention discloses a method for synthesizing 1-cyclopropyl-naphthyl by virtue of 1-vinyl-naphthyl and belongs to the technical field of organic synthesis. The method comprises the steps of under nitrogen protection and the condition of and ice bath, slowly dripping 1-vinylnaphthalene into tert-butylmagnesium chloride as a Grignard reagent with the concentration of 3M, wherein the molar ratio of the 1-vinylnaphthalene to the tert-butylmagnesium chloride is 1:(0.3-2); after dripping, adding 1-6mol methylenehalide and 0.3-2mol tert-butylmagnesium chloride as the Grignard reagent with the concentration of 3M, after reacting for 1-6 hours, quenching the reaction by virtue of a saturated ammonium chloride solution, extracting by virtue of ethyl acetate, evaporating the ethyl acetate by virtue of decompression, and carrying out recrystallization by virtue of methylbenzene to obtain a product. With the adoption of the method, with the 1-vinylnaphthalene and the methylenehalide as cheap chemical products as initial raw materials, the 1-cyclopropyl-naphthyl is synthesized by virtue of a one-step method; as the reaction conditions are mild and the operation is convenient, the method is suitable for industrial large scale production, and the synthesis cost of the 1-cyclopropyl-naphthyl is greatly lowered.
Owner:CHANGZHOU UNIV
Method for synthesizing 3-(tert-butyl)phenol
ActiveCN105198710ALow costRaw materials are cheap and easy to getOrganic compound preparationAmino compound preparationChlorobenzeneTert butyl phenol
The invention relates to a method for synthesizing 3-(tert-butyl)phenol. The method includes the following steps that 4-tert-butyl-1-chlorobenzene serves as a raw material and is nitrated to obtain 1-chlorine-4-tert-butyl-2-nitrobenzene; the 1-chlorine-4-tert-butyl-2-nitrobenzene is reduced to generate 3-tert-butylaniline; the 3-tert-butylaniline is diazotized and subjected to a hydrolysis reaction, and the 3-(tert-butyl)phenol is obtained. According to the path, the raw material is cheap and easy to get, elementary reaction conditions of the steps are mild, after-treatment is simple, the four-step reaction total yield is high, the 3-(tert-butyl)phenol with the purity higher than 97% can be obtained through distilling, and industrial production prospects are achieved.
Owner:RUDONG ZHONGYI CHEM
Method for preparing 5-tert butyl meta-xylene by adopting tubular reactor
InactiveCN109400434ATo address the effects of backmixing,Solve load problemsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTert-Butyl chlorideMeta-xylene
Owner:濮阳市欧亚化工科技有限公司
Preparation method of bis(ditert-butyl phenyl phosphine)palladium dichloride
ActiveCN110759951AHigh yieldGroup 8/9/10/18 element organic compoundsGrignard reagentTert-Butyl chloride
The invention discloses a preparation method of bis(ditert-butyl phenyl phosphine)palladium dichloride. The preparation method includes the following steps that (1) palladium sponge is dissolved in royal water, concentrated, saltpetre-removed and then filtered, and a filtrate is diluted with water to obtain a solution of palladium chloride acid; and then the filtrate is dropwise added into an ethanol solution of 1,5-cyclooctadiene, reacted until precipitation is no longer precipitated, and filtration is conducted to obtain 1,5-cyclooctadiene palladium chloride; (2) a phenyl grignard reagent isdropwise added into a tetrahydrofuran solution of bi tertiary butyl phosphorus dichloride to obtain a tetrahydrofuran solution of bis(ditert-butyl phenyl phosphine); (3) the tetrahydrofuran solutionof the bis(ditert-butyl phenyl phosphine) is dropwise added into a tetrahydrofuran solution of the1,5-cyclooctadiene palladium chloride; (4) filtration is conducted, and a filter cake is washed with anhydrous ethanol; and (5) drying is conducted. According to the preparation method, the 1,5-cyclooctadiene palladium chloride prepared by taking the palladium chloride acid as a metal source is used as a reaction precursor, the prepared bis(ditert-butyl phenyl phosphine) is used as an ligand, the ligand does not require purification, and the yield of products is high.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Method for preparing memantine hydrochloride
InactiveCN1193981CGood crystal formHigh purityNervous disorderPreparation by rearrangement reactionsMemantine HydrochlorideTert-Butyl chloride
The invention is a manufacturing method of diamante amine hydrochlorate. The invention adopts 1, 3-dimethyl adamantine to reacts with tert-butylchlorine and gets 1-chlorine-3, 5-dimethyl adamantine; then reacts with acetamide directly, the educt from water reacts with sodium hydroxide in ethanediol or glycerine solvent, extracts by acetic ester, condenses, and blow in dry hydrochloride gas and gets the coarse product. There gets the pure product through alcohol-acetic ester recrystallization.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Synthetic methods for nutame
InactiveCN100432097CSimple processSimple and fast operationDipeptidesFood preparationSynthesis methodsTert-Butyl chloride
This invention discloses a synthesis method for Neotame. The said method includes the following steps: (1) carrying out a reaction of tert-butanol with hydrochloric acid to obtain tert-butyl chloride; (2) adding catalyst in tert-butyl chloride, and charging ethylene gas and reacting to obtain 3, 3-dimethyl-1-chlorobutane; (3) oxidating 3, 3-dimethyl-1-chlorobutane to obtain 3, 3-dimethyl butyraldehyde, and purifying; and (4) carrying out a reaction of 3, 3-dimethyl butyraldehyde with aspartame to form imine, and catalytic-hydrogenating to obtain Neotame. The invention has the advantages of simple process, easy operation, low cost, and high purity of the product.
Owner:JINAN UNIVERSITY
Preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium
InactiveCN108659054AQuality improvementHigh yieldGroup 8/9/10/18 element organic compoundsGrignard reagentTert-Butyl chloride
The invention discloses a preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium. The preparation method comprises the following steps that 1, a Grignard reagent isprepared from the raw materials of N, N-dimethyl p-haloaniline and magnesium chips; 2, the Grignard reagent is taken and cooled, then a catalyst is added for reaction, then di-tert-butylchlorophosphane is dropwise added, and heating is carried out for reaction so as to obtain di-tert-butyl-4-dimethylaminophenylphosphine; 3, the di-tert-butyl-4-dimethylaminophenylphosphine is purified; and 4, complexing bis(acetonitrile)dichloropalladium and the purified di-tert-butyl-4-dimethylaminophenylphosphine so as to obtain a target product. The preparation method has the advantages that the di-tert-butyl-4-dimethylaminophenylphosphine is purified, the high-purity di-tert-butyl-4-dimethylaminophenylphosphine is used for reacting with the bis(acetonitrile)dichloropalladium, and therefore the yield loss of the noble metal palladium is greatly reduced, the preparation cost is greatly lowered, and the method has a good practical value.
Owner:陕西瑞科新材料股份有限公司
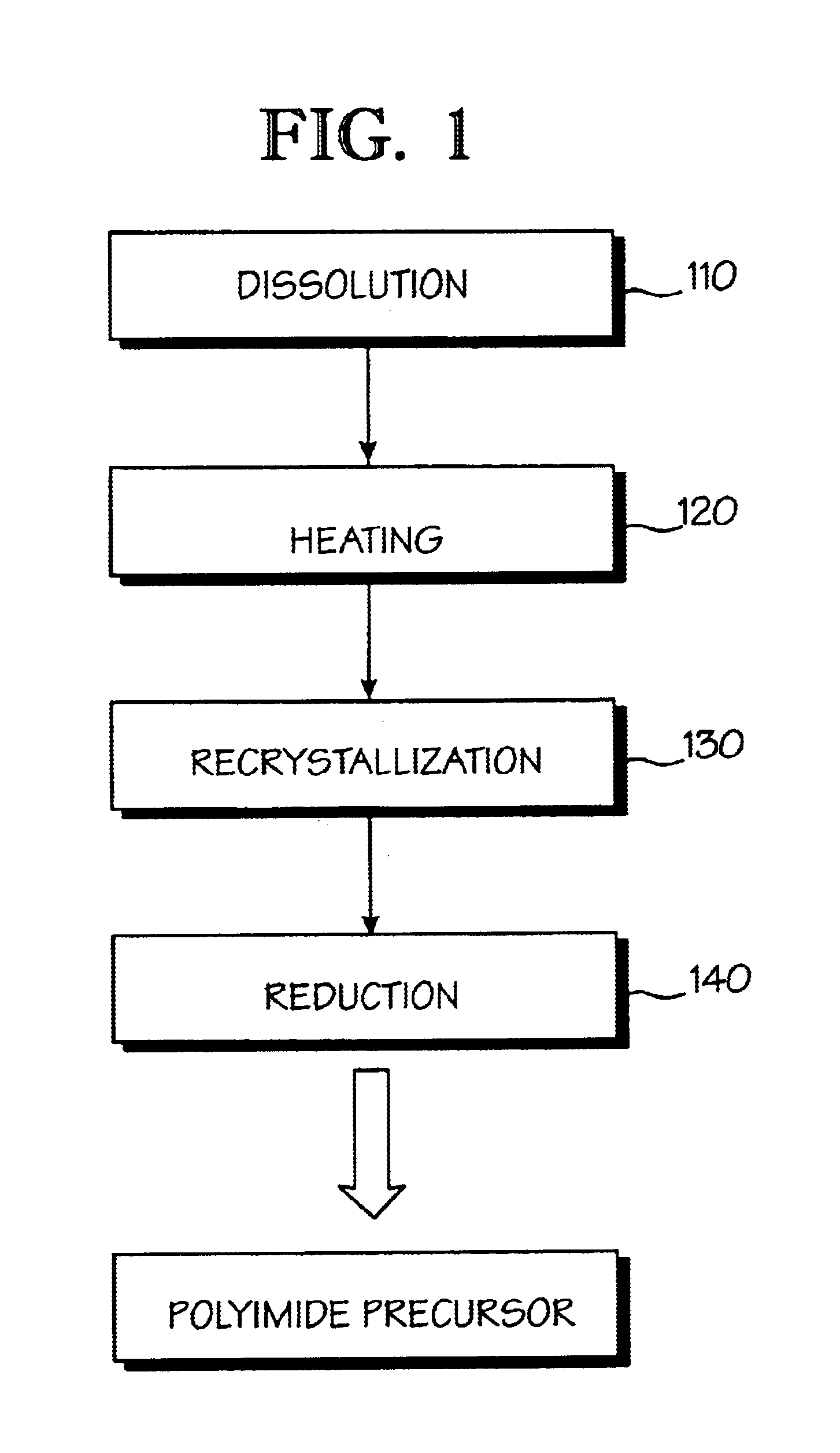
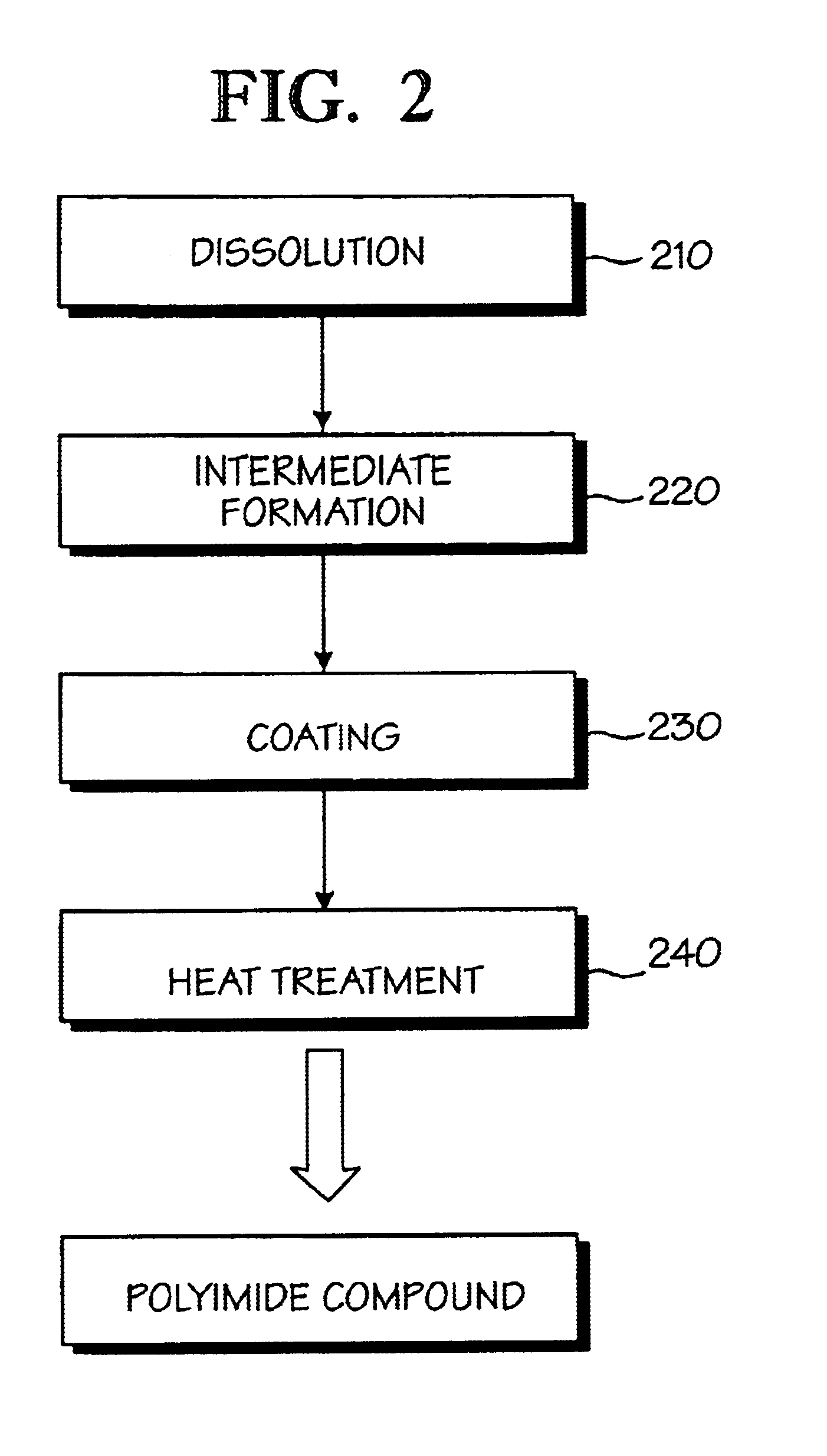
Optical polyimide precursor, optical polyimide compound and fabricating method thereof
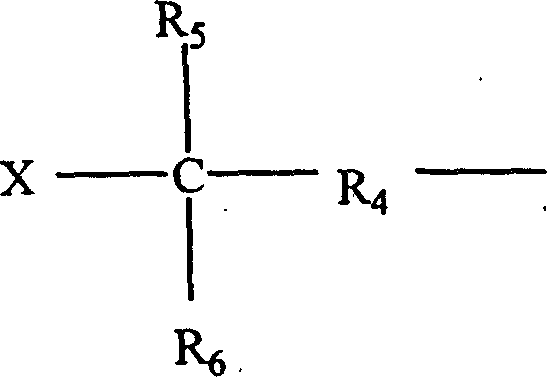
InactiveUS6891067B2Avoid crackingReduce absorption lossOrganic compound preparationDiaryl/thriaryl methane dyesNitro compoundAromatic moiety
The present invention provides an optical polyimide precursor for use in making a polyimide. The precursor is defined by the following formula: wherein X is Cl, Br, oxo-halide, or fully halogenated alkyl, and A is a divalent aromatic or halogenated aromatic moiety. The present invention provides a method of preparing a diamine compound for use as an optical polyimide precursor. The method includes the steps of dissolving 2-chloro-5-nitrobenzotrifluoride and a diol in N,N-dimethylacetamide to form a solution, adding potassium carbonate, tert-butylammonium chloride and copper powder to said solution and heating the resulting mixture, removing the copper, precipitating and recrystallizing a dinitro-compound resulting from heating the mixture, and dissolving the dinitro-compound and reducing the dinitro-compound to yield a diamine compound.
Owner:SAMSUNG ELECTRONICS CO LTD
Method for the synthesis of low cost initiators for telechelic polyisobutylenes
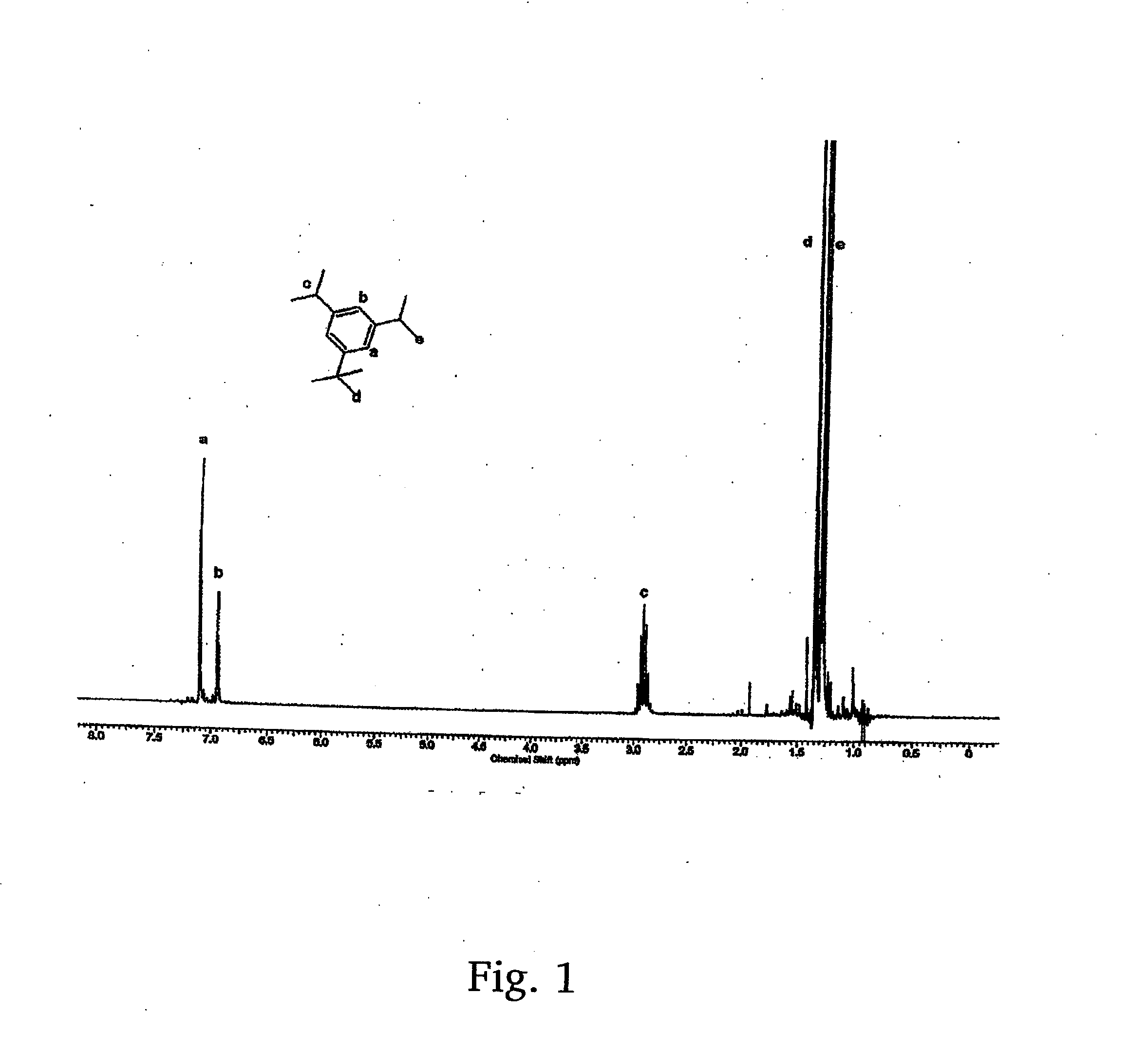
ActiveUS20140235905A1Low costHighly desirableOrganic compound preparationCatalystsPtru catalystIsopropyl
A method for the synthesis of 1,3-di(chloropropyl)-5-tert-butyl benzene includes the steps of conducting Friedl-Crafts alkylation of 1,3-diisopropyl benzene by tert-butyl chloride in the presence of an alkylation catalyst to obtain 1-tert-butyl-3,5-diisopropylbenzene; peroxidizing the 1-tert-butyl-3,5-diisopropyl benzene by gaseous oxygen in the presence of a peroxidation catalyst in a basic solution to obtain 1,3-di(peroxypropyl)-5-tert-butylbenzene; reducing the 1,3-di(peroxypropyl)-5-tert-butylbenzene with a reducing agent to 1,3-di(hydroxyl propyl)-5-tert-butylbenzene; and chlorinating the 1,3-di(hydroxypropyl)-5-tert-butylbenzene to obtain 1,3-di(chloropropyl)-5-tert-butyl benzene.
Owner:THE UNIVERSITY OF AKRON
Preparation method of tonalide intermediate HMT
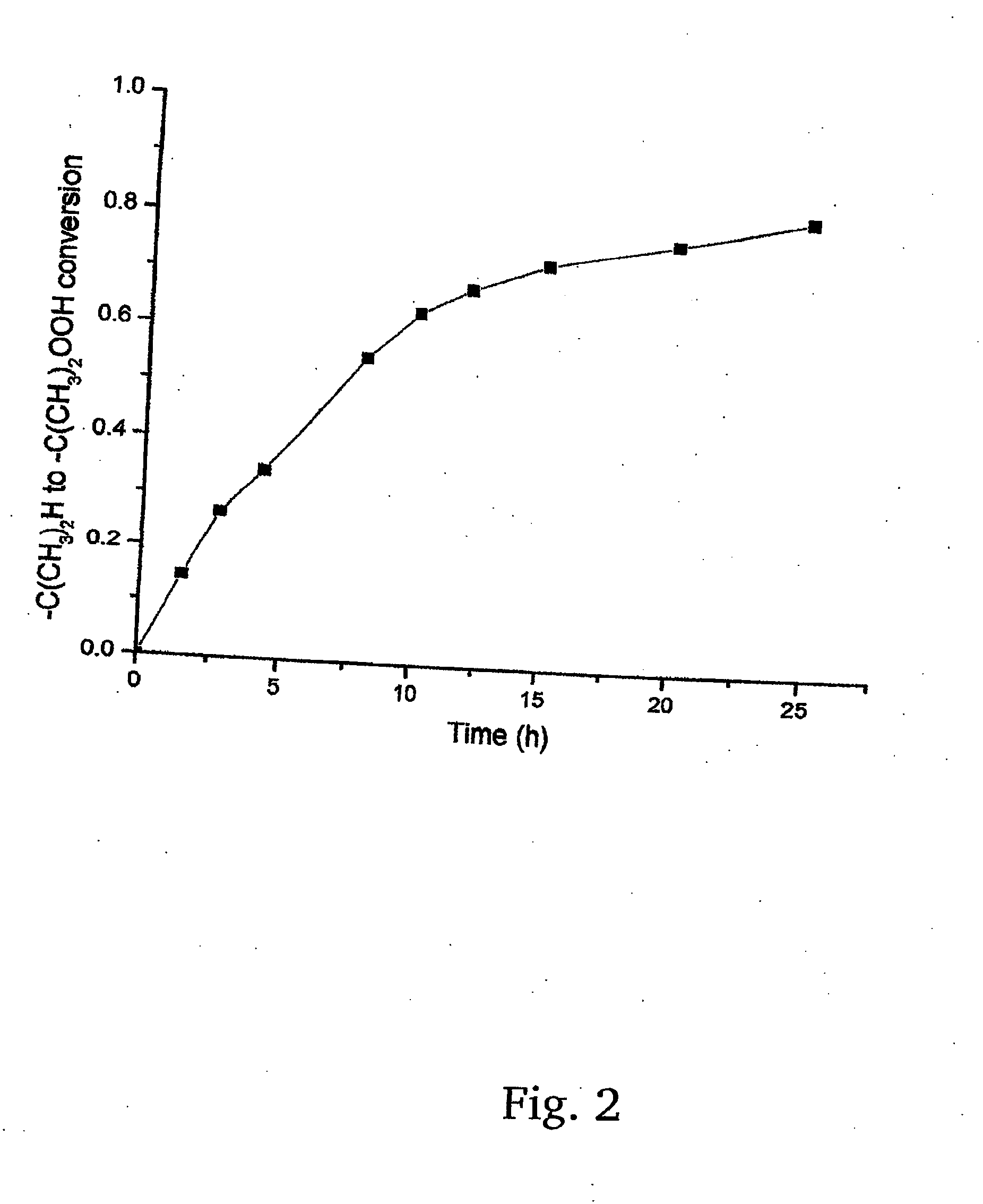
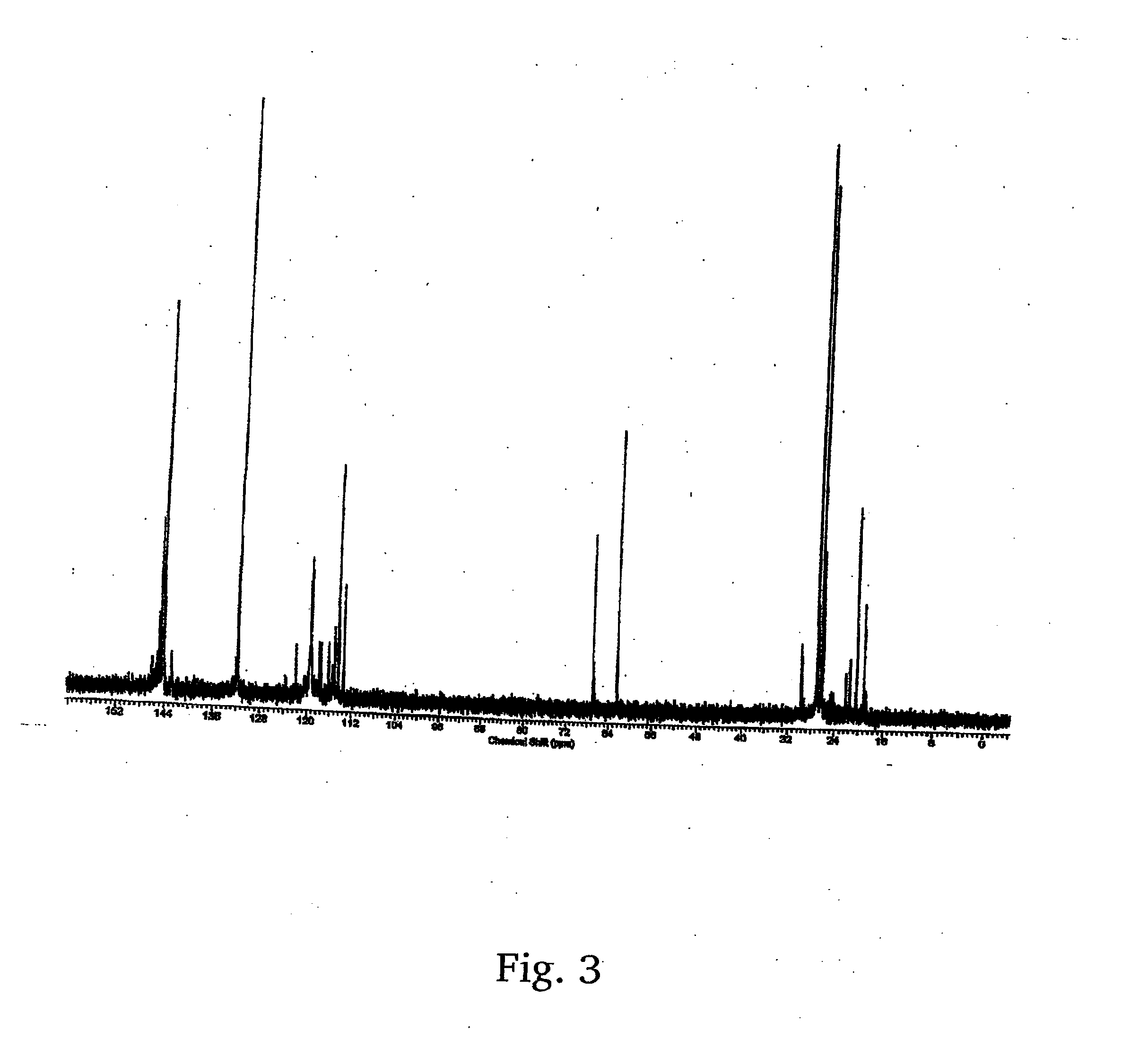
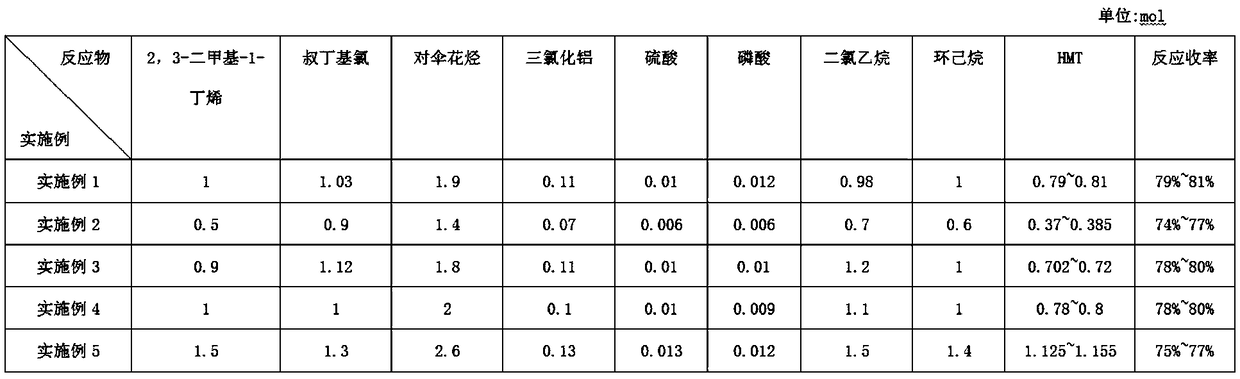
InactiveCN109369317AHigh yieldChanging the method of recrystallizationDistillation purification/separationHydrocarbonsPurification methodsPhosphoric acid
The invention provides a preparation method of a tonalide intermediate HMT. The preparation method comprises the steps of mixing p-cymene, sulfuric acid, phosphoric acid and aluminum trichloride in acertain ratio in a reactor; then mixing and dripping 2,3-dimethyl-1-butene and tert-butyl chloride into the reactor, and reacting at a room temperature for 1 hour to generate the tonalide intermediateHMT, wherein the molar ratio of 2,3-dimethyl-1-butene to tert-butyl chloride to p-cymene to aluminum trichloride to sulfuric acid to phosphoric acid to dichloroethane to cyclohexane is 0.5-1.5 to 0.9-1.3) to 1.4-2.6 to 0.07-0.13 to 0.006-0.013 to 0.006-0.012 to 0.7-1.5 to 0.6-1.4; and directly transferring the reacted substances to a rectification column for rectification and purification to obtain a tonalide intermediate HMT product. The HMT product with higher yield can be obtained from new reaction substances, the purification method is improved, and the conventional recrystallization purification method is changed to reduce pollution and lower the production cost.
Owner:江西开源香料有限公司
Method for preparing bis(4-tert-butyl benzyl)sulfide product
ActiveCN102924349AReduce surface tensionImprove the state of the interfaceSulfide preparationPtru catalystTert-Butyl chloride
The invention relates to a method for preparing a bis(4-tert-butyl benzyl)sulfide product, belonging to the technical field of preparation of medical intermediate compounds and polymer antioxidants. The method comprises the following step: in the presence of both an emulsifier and a phase-transfer catalyst, reacting 4-tert-butyl benzyl chloride and a sodium sulfide water solution used as raw materials in an organic solvent-water system, wherein the proportioning of the raw materials is as follows: the mol ratio of the 4-tert-butyl benzyl chloride to the sodium sulfide is 1:(0.5-0.9); the mass ratio of the 4-tert-butyl benzyl chloride to the organic solvent is 1:(0.8-1.6); the mass ratio of the 4-tert-butyl benzyl chloride to the emulsifier is 1:(0.008-0.033); the mass ratio of the 4-tert-butyl benzyl chloride to the phase-transfer catalyst is 1:(0.008-0.033); the reaction temperature in the reaction process is 50-70 DEG C; and the reaction time is 150-240 minutes. Thus, high raw material conversion rate and product yield are achieved.
Owner:山东正鑫新能源有限公司
Method for preparing gamma-tertiary butyl-chlorobenzene butanone
InactiveCN101700996AEasy to absorb moistureReaction is easy to controlOrganic compound preparationCarbonyl compound preparationChlorobenzeneButyl chloride
The invention relates to a method for preparing gamma-tertiary butyl-chlorobenzene butanone. The method is characterized by comprising the following steps of: (1) acylation reaction: sucking butyrolactone into a clean and dry reaction kettle and strring, and then adding zinc chloride into the reaction kettle for reaction to generate semi-finished butyl chloride; (b) hydrolysis reaction: adding dichloroethane and aluminium choride into a clean and dry reaction kettle, dropwise adding the butyl chloride into the reaction kettle while stirring; then continuing to dropwise adding tedin benzene , and steaming off dichloroethane to generate the gamma-tertiary butyl-chlorobenzene butanone. 3gamma-tertiary butyl-chlorobenzene butanone products prepared by the method are pale yellow granules, wherein the content of the 3gamma-tertiary butyl-chlorobenzene butanone is more than or equal to 90 percent, and the pH value is larger than or equal to 3.85. The reaction process of the method is easy to control, the utilization rate of raw materials and the yield of the products are improved, and the method conforms to the requirements on environmental protection and cleaner production and improves the economic benefits of enterprises.
Owner:扬州市天平化工厂有限公司
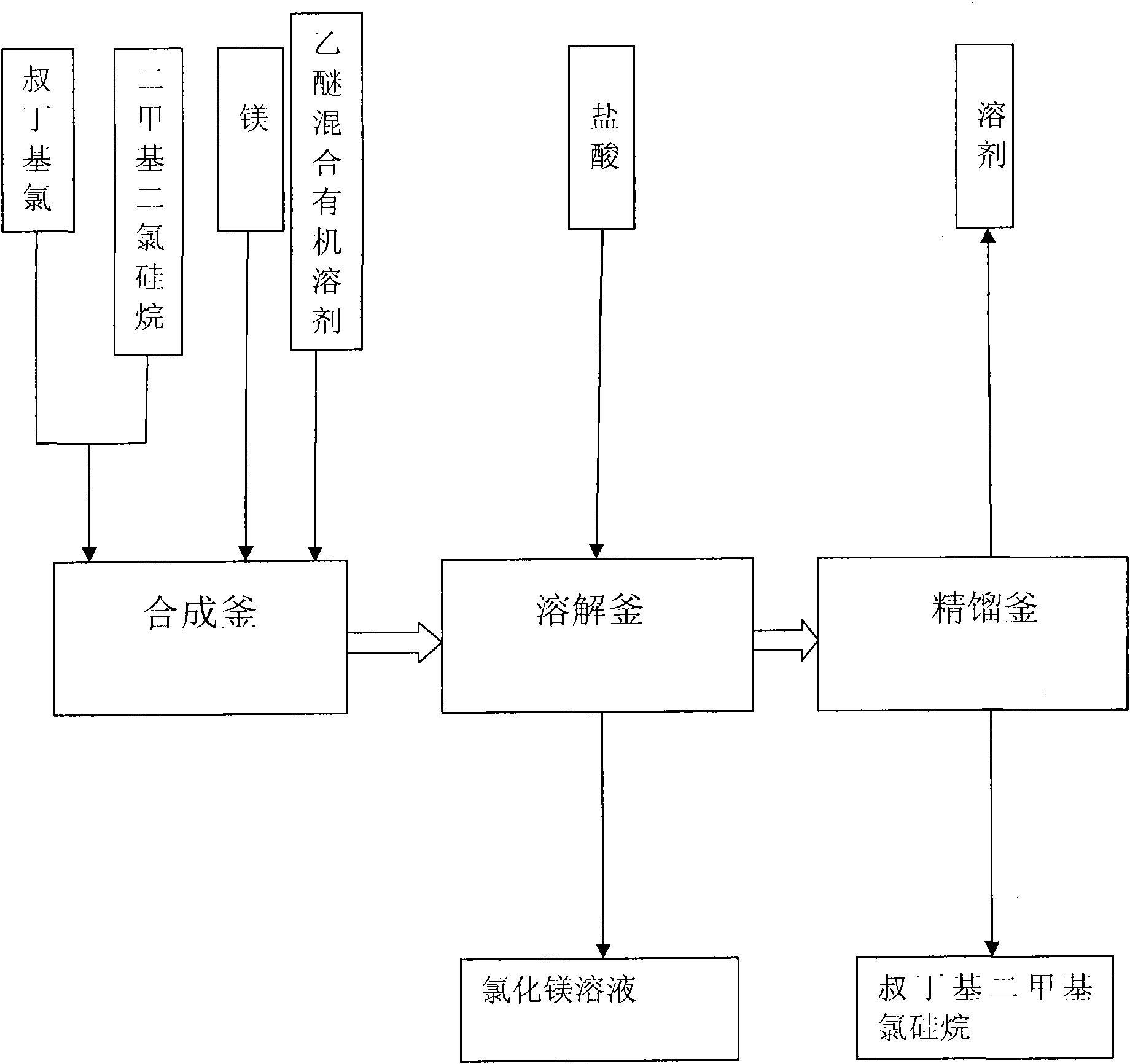
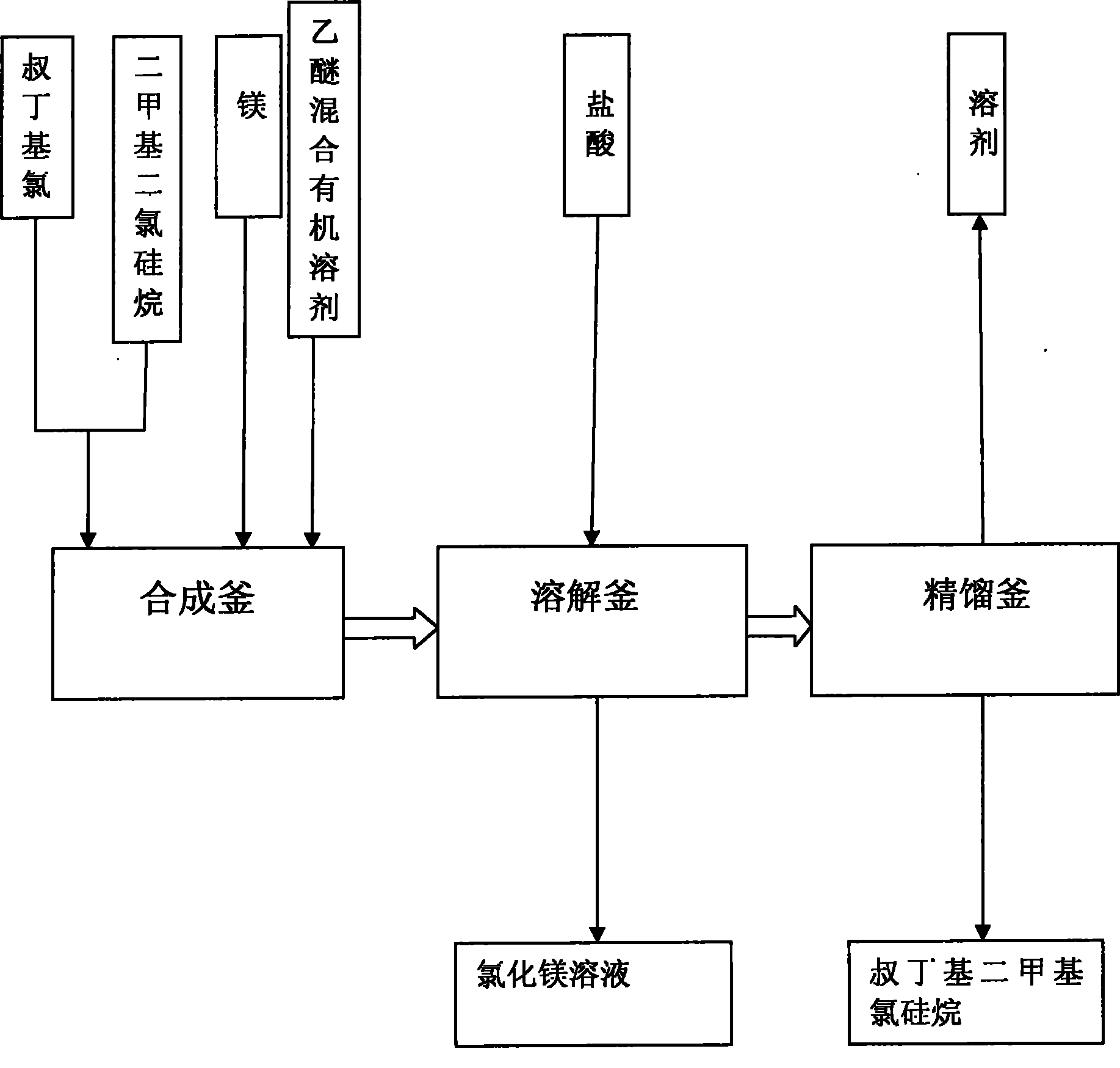
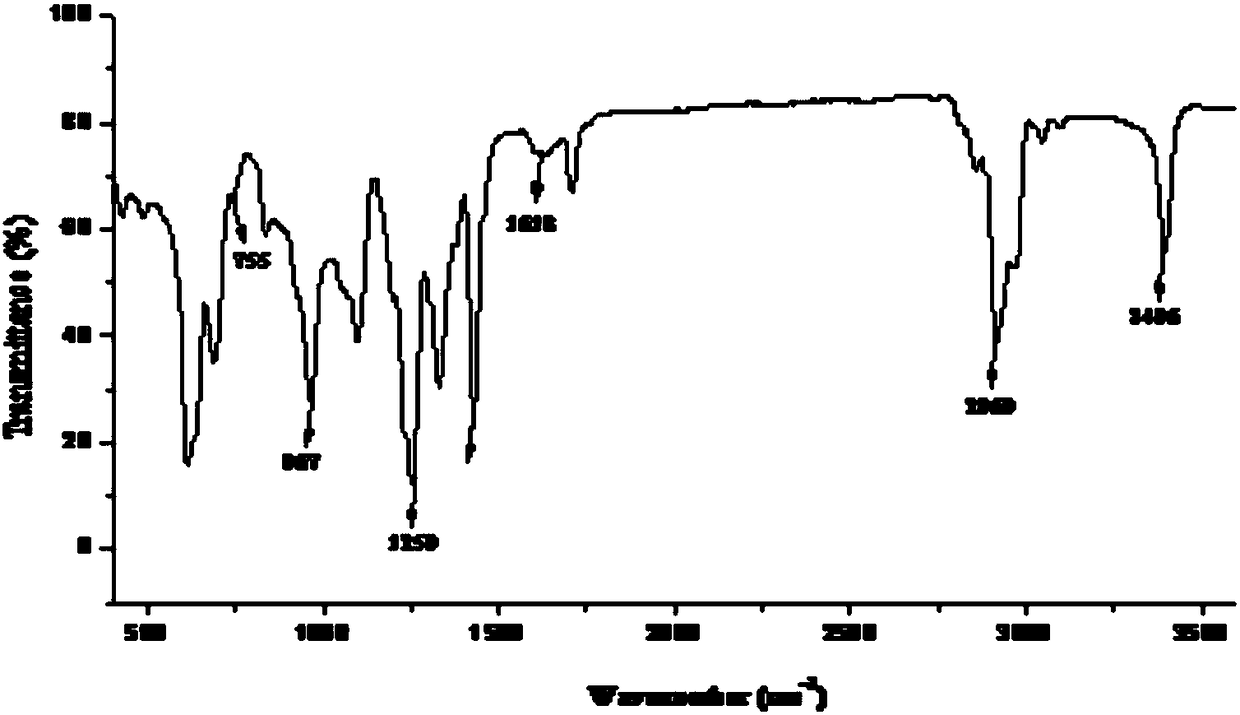
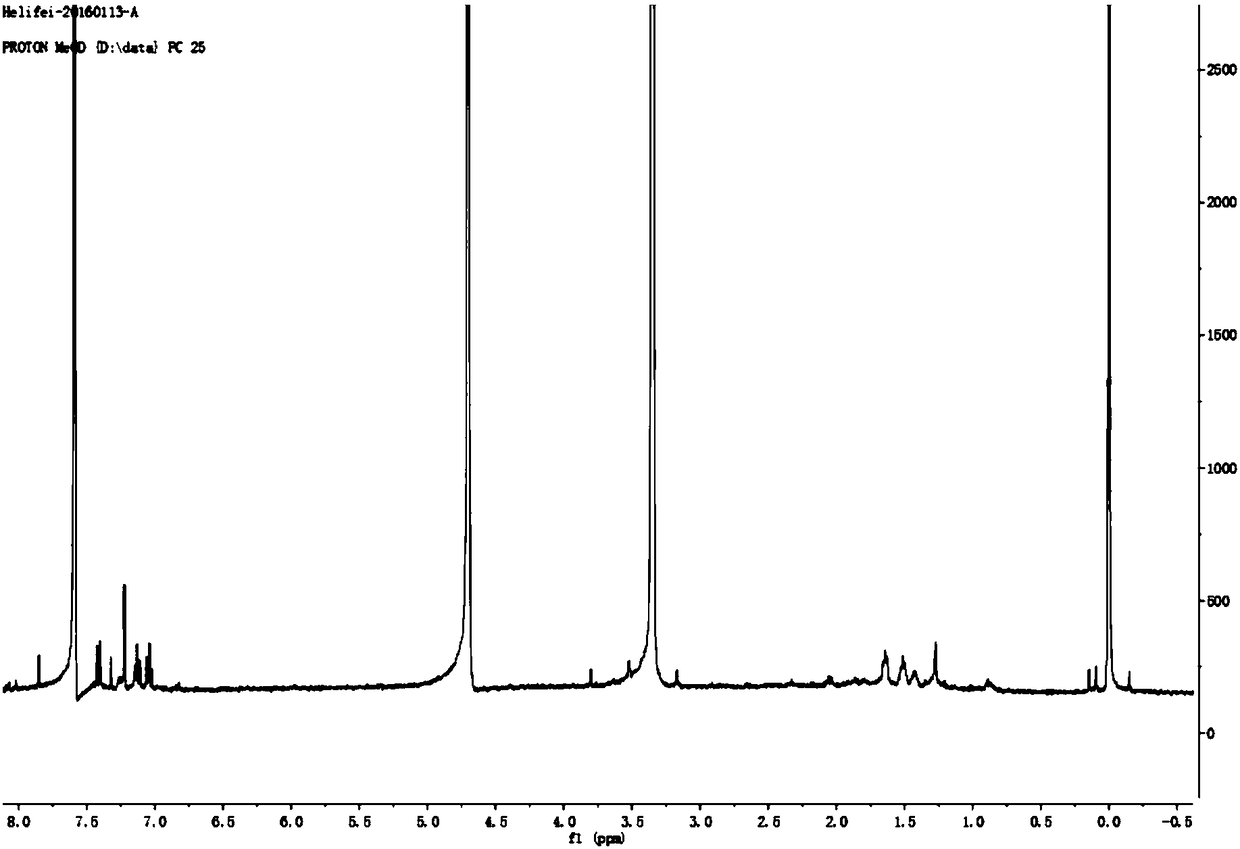
Method for preparing tert-butyldimethyl chlorosilane
InactiveCN101817842AEasy to operateEasy to controlGroup 4/14 element organic compoundsButyl chlorideDissolution
The invention relates to a method for preparing tert-butyldimethyl chlorosilane, which is characterized by comprising the following steps of: a, in a synthesis kettle, putting 10 to 15 weight parts of magnesium into 160 to 240 volume parts of mixed solvent consisting of ether and cyclohexane, dripping mixed solution consisting of 35 to 55 weight parts of tertiary butyl chloride and 50 to 78 weight parts of dimethyl dichlorosilane into the mixed solvent at the temperature of between 40 and 55 DEG C, a preserving heat for 2.5 to 3.5 hours after the dripping is finished to obtain a synthesized material; b, transferring the synthesized material to a dissolution kettle, cooling the synthesized material to between 10 and 15 DEG C, then dripping 400 to 600 volume parts of 25 to 30 percent hydrochloric acid into the dissolution kettle, standing and demixing the solution, and removing wastewater at the bottom to obtain upper liquor; and c, transferring the upper liquor in the dissolution kettle to a rectification kettle to perform purification, and removing the solvent to obtain a tert-butyldimethyl chlorosilane product. The method has the advantages of simple synthesis process, high safety and stable product yield.
Owner:HAIMEN BEST FINE CHEM
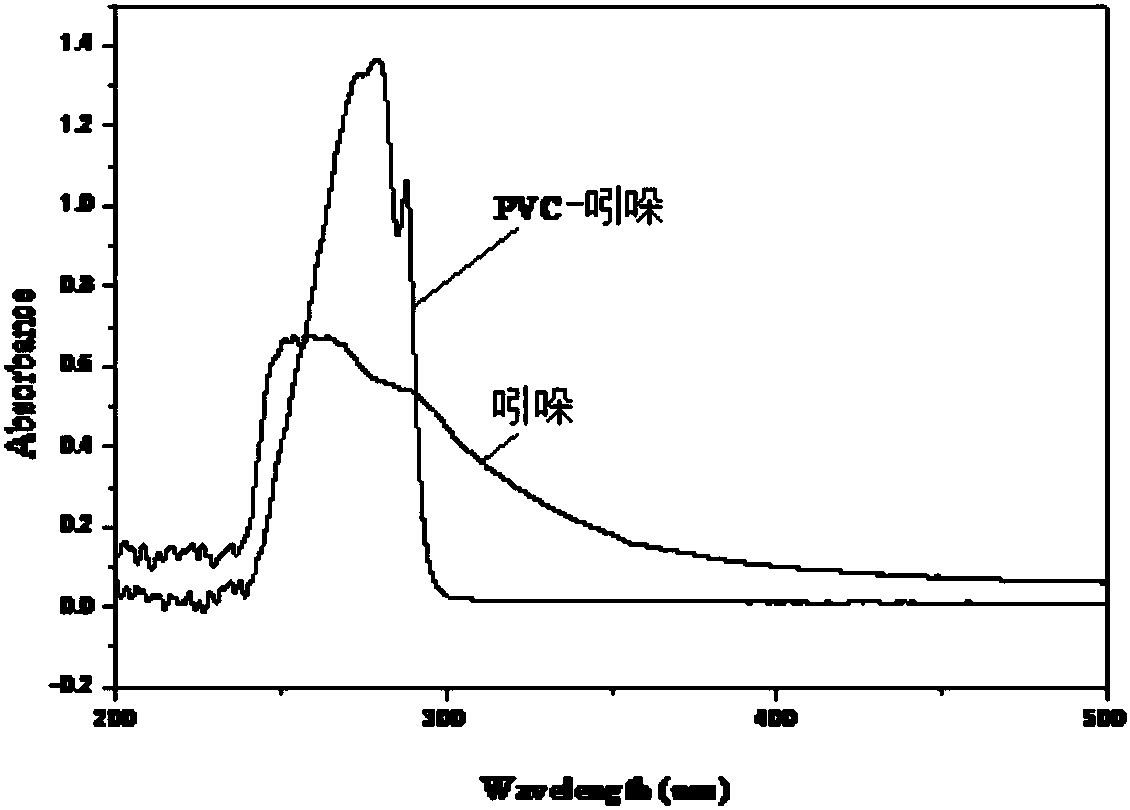
PVC (polyvinyl chloride) functionalized master batch, preparation method and PVC-indole grafted blended product
The invention relates to PVC (polyvinyl chloride) functionalized master batch, a preparation method and a PVC-indole grafted blended product. The structure of the master batch is shown in the description. In order to improve the performance of PVC and overcome the defect that tert-butyl chloride, allyl chloride and the like exist in PVC macromolecular chains, a chemical grafting reaction is adopted, an indole modified PVC (PVC / indole) material with good redox and fluorescence functions and better thermal stability is prepared from aromatic indole micromolecules through an Friedel-Crafts alkylation reaction, and the stabilization and functionalization purposes of PVC are achieved through replacement of active chlorine with aromatic rings.
Owner:XIAN UNIV OF SCI & TECH
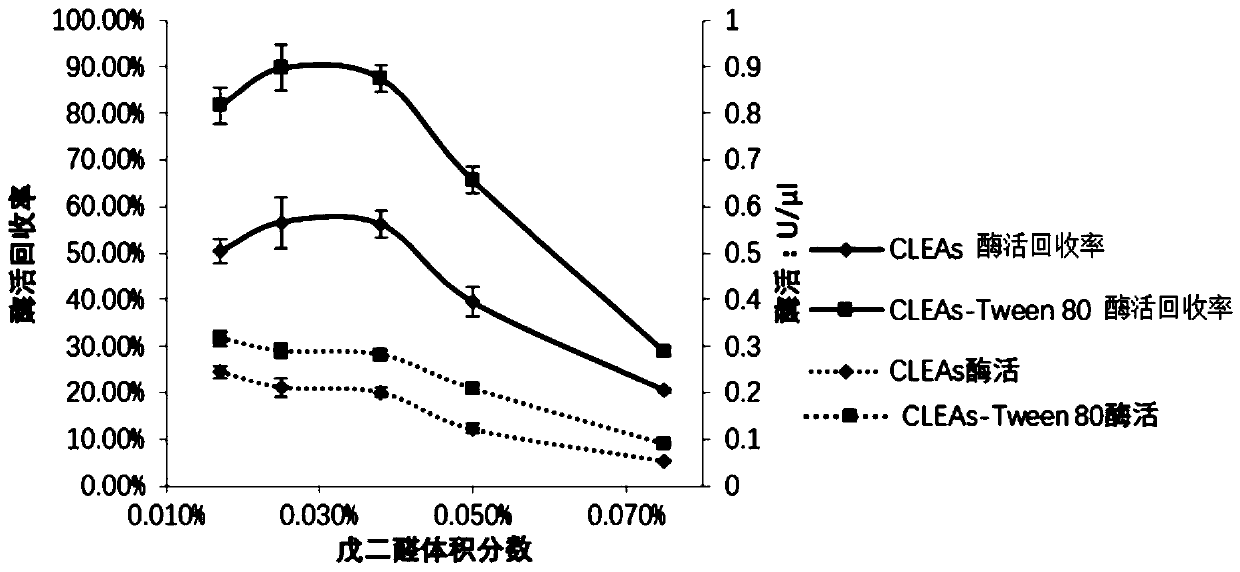
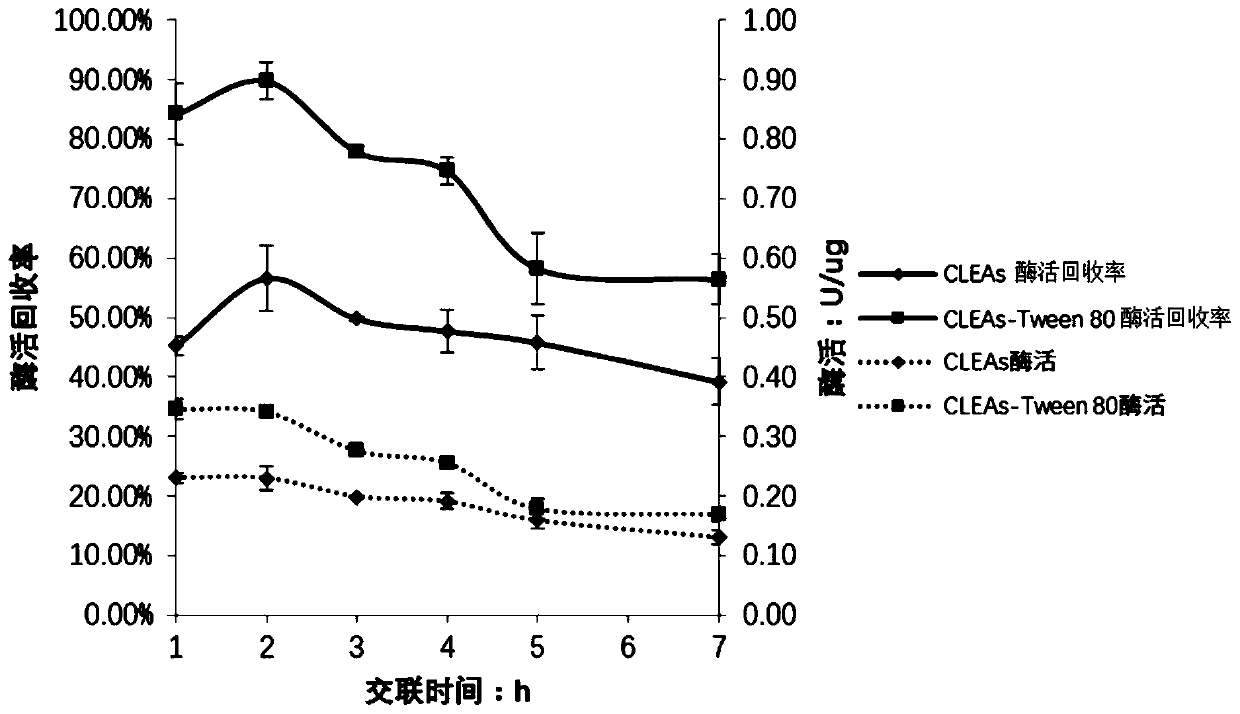
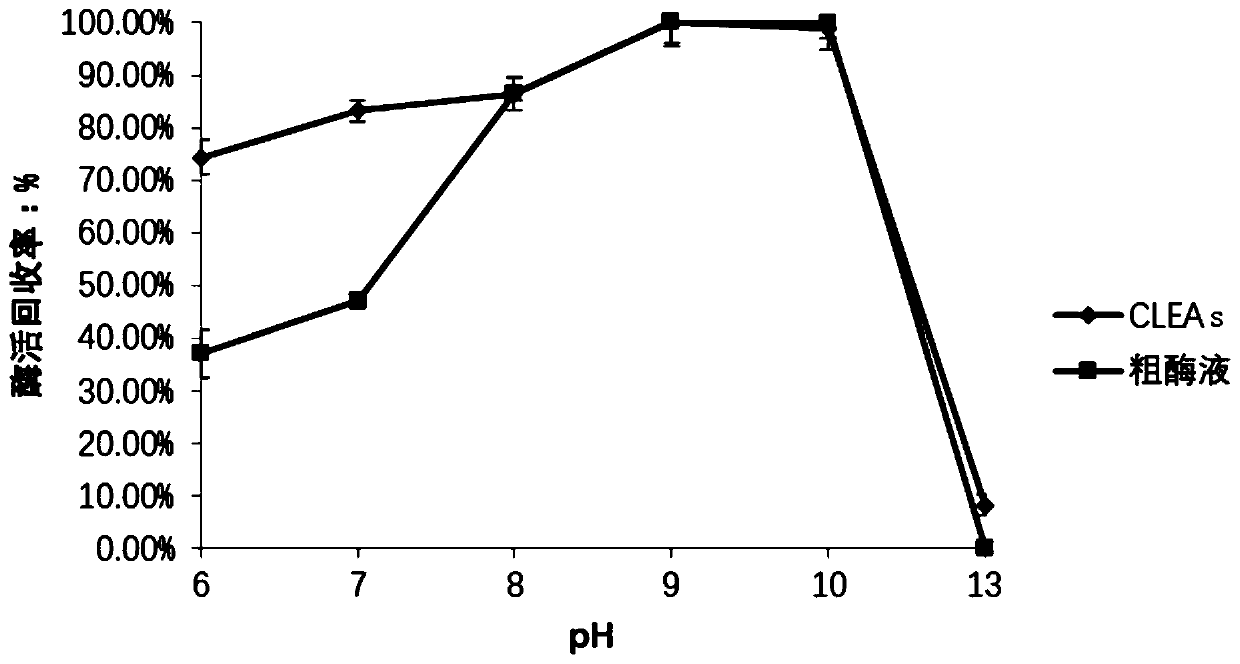
Method for catalyzing synthesis of atazanavir intermediate by carbonyl reductase CLEAs
InactiveCN109988789ASimple reaction systemEasy to purifyOrganic chemistryOxidoreductasesCarbamateReaction temperature
The invention relates to the field of medicine, in particular to a method for catalyzing synthesis of an atazanavir intermediate by a carbonyl reductase CLEAs. The method uses (S)-tert-butyl (4-chloro-3-carbonyl-1-phenylbutyl-2-yl) carbamate as a substrate and a carbonyl reductase NaSDR crosslinked aggregate as a catalyst, and the catalyzing synthesis of tert-butyl ((2S, 3R)-4-chloro-3-hydroxy-1-phenylbutyl-2-yl)) carbamate is carried out in a system in which a co-substrate, a co-solvent and a buffer solution are present. By the adoption of the method for the catalyzing synthesis of the atazanavir intermediate by the carbonyl reductase CLEAs, a reaction system is simpler, additional expensive coenzymes are not necessary, and glucose is not required to participate in a coenzyme cycle; in addition, the reaction temperature is closer to room temperature; the carbonyl reductase NaSDR crosslinked aggregate as a biocatalyst has better temperature stability and pH stability and can be reusedin 6 batches, so that the method is more energy-saving and environmentally friendly, and cost is further reduced.
Owner:SHANGHAI UNIV OF MEDICINE & HEALTH SCI
Method for synthesizing 4-tert-butyl benzyl chloride
InactiveCN102050697AReduce pollutionImprove conversion rateHalogenated hydrocarbon preparationWater bathsTert-Butyl chloride
The invention discloses a method for synthesizing 4-tert-butyl benzyl chloride. The method comprises the following steps of: (1) weighing a certain amount of tertiary butyl, formaldehyde, hydrochloric acid and formic acid, and mixing the raw materials with stirring; (2) heating the mixed raw materials in water bath to 60 to 70 DEG C, controlling the reaction temperature to be 70 to 75 DGE C and reacting for 10 and 20 hours; (3), cooling to room temperature, separating out rough products, washing with water, 8 weight percent K2CO3, water and diethyl ether in turn, and adding anhydrous calcium carbonate for drying; and (4) distilling under normal pressure and then distilling under reduced pressure to obtain the 4-tert-butyl benzyl chloride. In the method, the conversion rate of reaction is high and is about 50 to 60 percent, yield is kept in a high level and is more than 70 percent, the operation process is simple, and the method is environmental-friendly, and has obvious economic benefit, social benefit and market competitiveness.
Owner:李莉
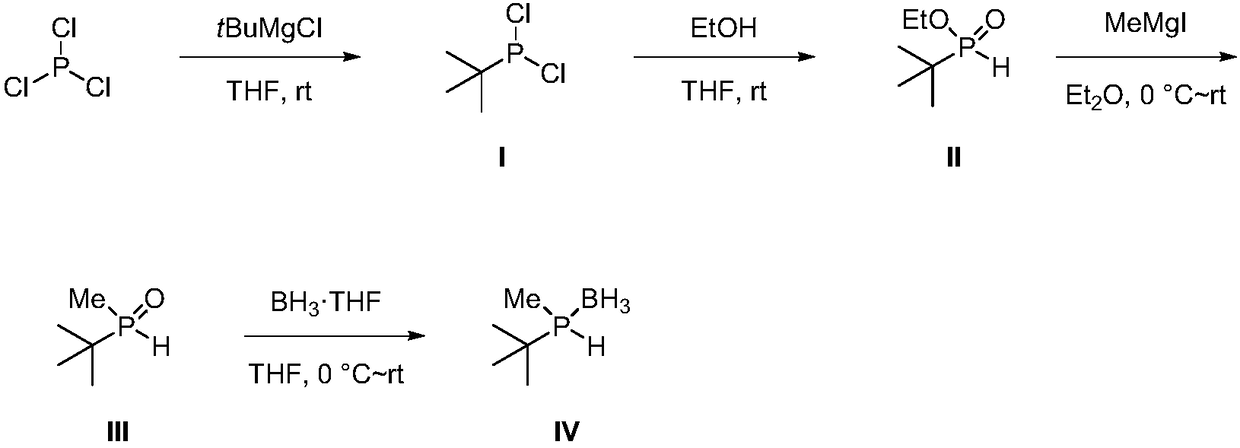
Phosphorus chiral important intermediate preparation method
ActiveCN108409784AShort process routeSimple and fast operationGroup 5/15 element organic compoundsGrignard reagentTert-Butyl chloride
The invention discloses a phosphorus chiral important intermediate preparation method, which comprises: carrying out a substitution reaction on phosphorus trichloride and tert-butyl magnesium chlorideto obtain tert-butyl phosphine dichloride I; carrying out an esterification reaction on the tert-butyl phosphine dichloride I and ethanol to obtain tert-butyl ethyl phosphite II; carrying out a methylation reaction on the tert-butyl ethyl phosphite II under the action of a methyl Grignard reagent methyl magnesium iodide to generate methyl tert-butyl phosphine oxide III; and obtaining a methyl tert-butyl phosphine hydrogen compound VI protected with borane through a borane reduction one-pot method. According to the present invention, the method has advantages of low-cost and easily-available raw material, short route, simple operation and good atomic economy, and provides the simple and easy-performing route for the preparation of the borane methyl tert-butyl phosphine hydrogen.
Owner:SHANGHAI JIAO TONG UNIV
Elastomeric compositions
InactiveCN1602320AWithout separate inflatable insertsWith separate inflatable insertsElastomerPolymer science
Owner:EXXONMOBIL CHEM PAT INC
Equipment for producing liquid composition as well as preparation method and application thereof
ActiveCN114716415AFully automatedThe technical means are clearIsotope introduction to heterocyclic compoundsEthoxidinePyridazine
The invention provides equipment for producing a liquid composition of 2-tert-butyl-4-chloro-5-((3-((4-((2-(2-fluoro [18F] ethyoxyl) ethyoxyl) methyl)-1H-1, 2, 3-triazole-1-yl) methyl) benzyl) oxygen) pyridazine-3 (2H)-one (hereinafter referred to as' Compound I '), comprising: a pretreatment module for enriching < 18 > F ions; the reaction module is used for reacting the enriched < 18 > F ions with a compound I precursor to obtain a compound I crude product; the purification module is used for purifying the compound I crude product to obtain a compound I pure product; and the prescription module is used for enriching and formulating the purified compound I pure product into a compound I liquid composition. The liquid composition of the compound I prepared by the equipment provided by the invention can be directly used clinically. The invention also provides a method for preparing the compound I liquid composition by the equipment and application of the equipment in preparation of the compound I liquid composition.
Owner:BEIJING SINOTAU INT PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
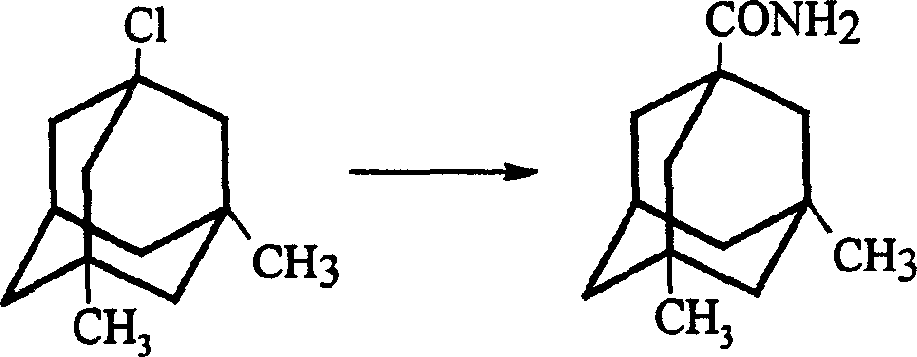
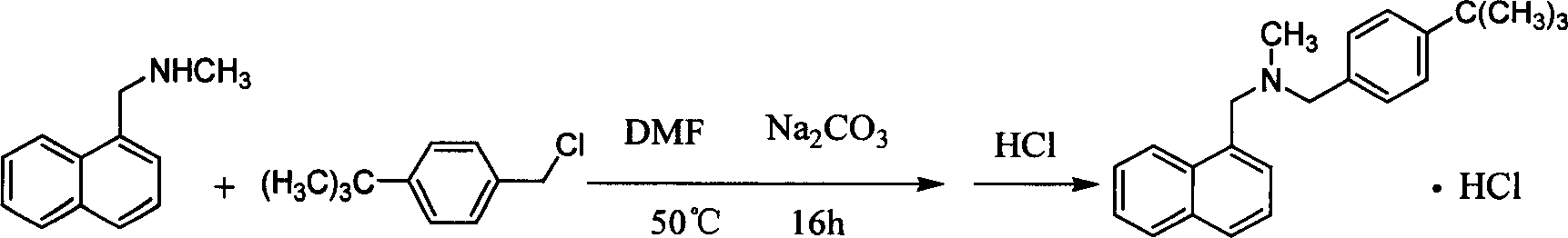
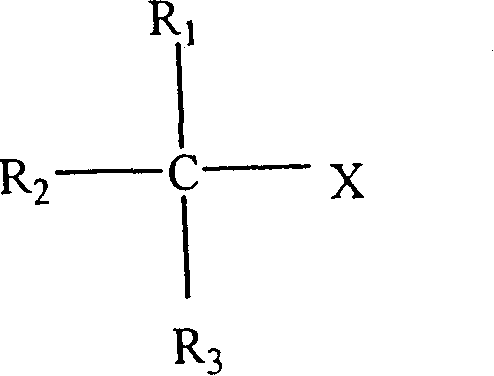
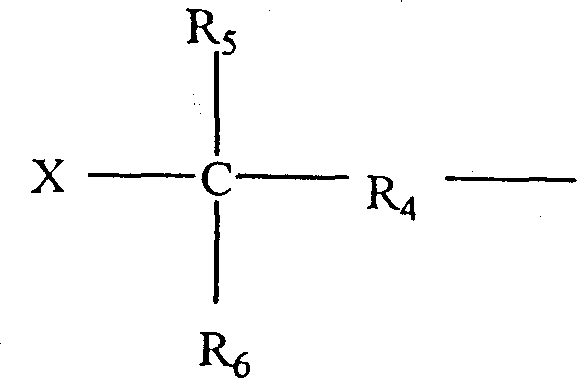
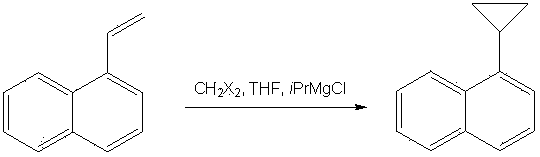
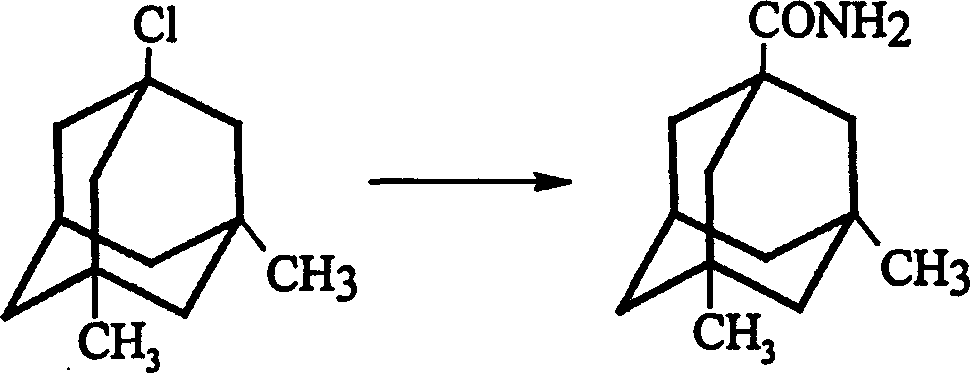
![Preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium Preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium](https://images-eureka.patsnap.com/patent_img/5b53075f-1439-43b2-b289-40a445d90dcf/HDA0001732131300000011.png)
![Preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium Preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium](https://images-eureka.patsnap.com/patent_img/5b53075f-1439-43b2-b289-40a445d90dcf/HDA0001732131300000021.png)
![Preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium Preparation method for bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]dichloropalladium](https://images-eureka.patsnap.com/patent_img/5b53075f-1439-43b2-b289-40a445d90dcf/HDA0001732131300000031.png)