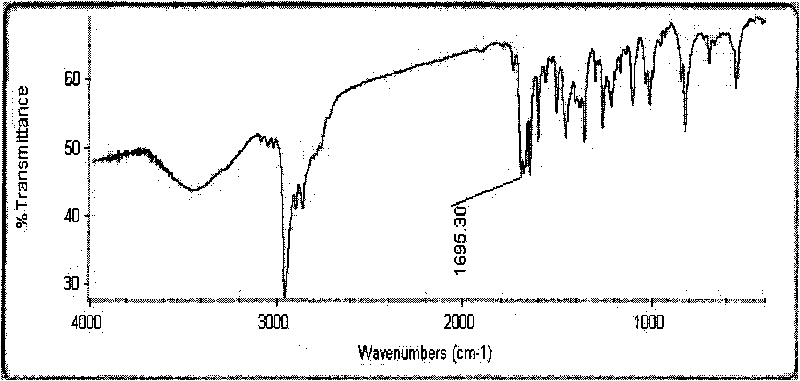
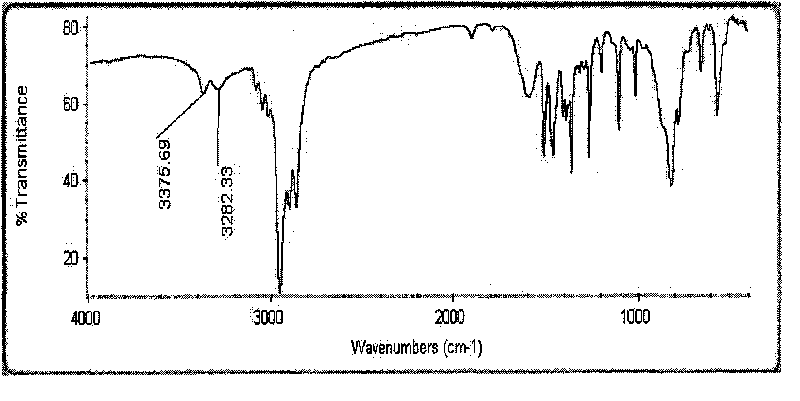
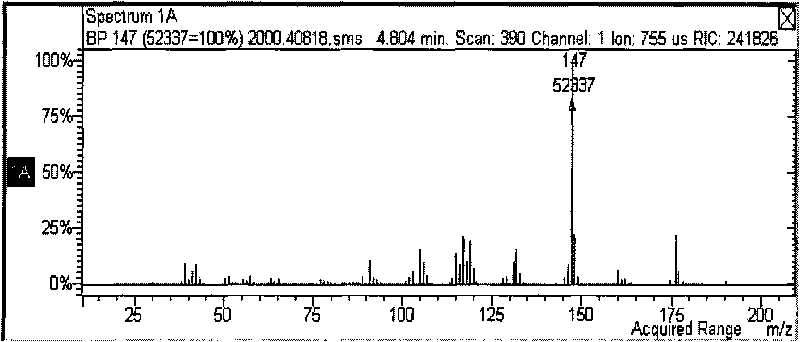
Method for preparing p-tert-butylbenzylamine
A technology of p-tert-butylbenzylamine and p-tert-butylbenzyl chloride, applied in the field of compound preparation, can solve the problems of inability to obtain tert-butylbenzylamine and low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 (control test, does not carry out secondary hydrolysis)
[0017] Add 5.7g of urotropine and 15ml of chloroform into a 250ml three-necked flask, set up a condensation reflux device, a thermometer, and stir at room temperature. The wall of the bottle was washed with chloroform, and the reaction was refluxed at 62° C. for 1 h. After the reaction is complete, a large amount of white solid precipitates are produced, cooled to room temperature, and suction filtered to obtain the white solid, which is the hexatropine quaternary ammonium salt of p-tert-butylbenzyl chloride prepared by the present invention;
[0018] The white solid obtained in the above steps is transferred to a 250ml three-necked flask, 15ml of concentrated hydrochloric acid is added thereto, it is dissolved under stirring, and then 45ml of ethanol with a concentration of 95% is slowly added, a large amount of white solid precipitates appear, and continue to dissolve at 80 Reflux reaction at ℃ fo...
Embodiment 2
[0020] Embodiment 2 (the present invention)
[0021] Add 5.7g of urotropine and 15ml of chloroform into a 250ml three-necked flask, set up a condensation reflux device, a thermometer, and stir at room temperature. After the urotropine is fully dissolved, add 6.1g of p-tert-butylbenzyl chloride at one time , and then rinse the bottle wall with 5ml chloroform, and heat and reflux at 62°C for 1h. After the reaction is completed, a large amount of white solid precipitates are produced, cooled to room temperature, and the white solid is obtained by suction filtration, which is the quaternary ammonium salt of tert-butyl benzyl chloride. ;
[0022] The white solid obtained in the above steps is transferred to a 250ml three-necked flask, 15ml of concentrated hydrochloric acid is added thereto, it is dissolved under stirring, and then 45ml of ethanol with a concentration of 95% is slowly added, a large amount of white solid precipitates appear, and continue to dissolve at 80 Reflux at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com