Preparation method of ZrO2 nanotube supported B2O3 catalyst
A technology of catalysts and nanotubes, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of complex catalyst preparation process and large amount of waste liquid, and achieve favorable performance and reaction The effect of faster speed and controllable pipe diameter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
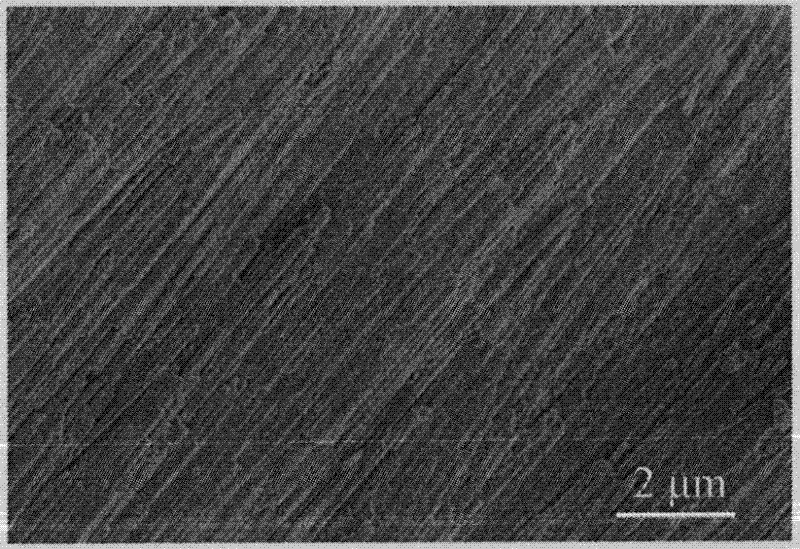
[0024] Formulated with 1.0% (wt) NH 4 F, 2% (wt) boric acid, 10% (wt) (NH 4 ) 2 SO 4 The aqueous solution is used as the reaction medium, the zirconium sheet is used as the anode, and the platinum sheet is used as the cathode. The distance between the anode and the cathode is 1.5 cm. The anodic oxidation reaction is carried out at room temperature with 20V direct current for 4 hours, and oxides are formed on the surface of the zirconium sheet. After the reaction, the oxide is dried at 120°C and calcined at 500°C to obtain the catalyst.
[0025] From attached figure 1 It can be seen that the diameter of the catalyst is 120nm, the length reaches 50μm, and the specific surface area is 24.3m 2 / g, which is similar to the specific surface area of a powder with a diameter of 42nm, and its volume is 14,600 times that of the powder.
Embodiment 2
[0027] Formulated with 0.5% (wt) NH 4 F, 6% (wt) ammonium borate, 5% (wt) (NH 4 ) 2 SO 4 The aqueous solution is used as the reaction medium, the zirconium sheet is used as the anode, and the platinum sheet is used as the cathode. The distance between the anode and the cathode is 1.5 cm. The anodic oxidation reaction is carried out at room temperature with 30V direct current for 3 hours, and oxides are formed on the surface of the zirconium sheet. After the reaction, the oxide is dried at 120°C and calcined at 400°C to obtain the catalyst.
Embodiment 3
[0029] Formulated with 1.0% (wt) NH 4 F, 0.1% (wt) boric acid, 12% (wt) (NH 4 ) 2 SO 4 The aqueous solution is used as the reaction medium, the zirconium sheet is used as the anode, and the platinum sheet is used as the cathode. The distance between the anode and the cathode is 1.5 cm. The anodic oxidation reaction is carried out at room temperature with 10V direct current for 5 hours, and oxides are formed on the surface of the zirconium sheet. After the reaction, the oxide is dried at 120°C and calcined at 600°C to obtain the catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com