Novel technology for synthesizing bactericide tebuconazole without solvent
A technology of tebuconazole and fungicide, which is applied in the new process field of solvent-free synthetic fungicide tebuconazole, can solve the problems of high recovery energy consumption and high boiling point of solvent, and achieve high equipment utilization, high yield and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
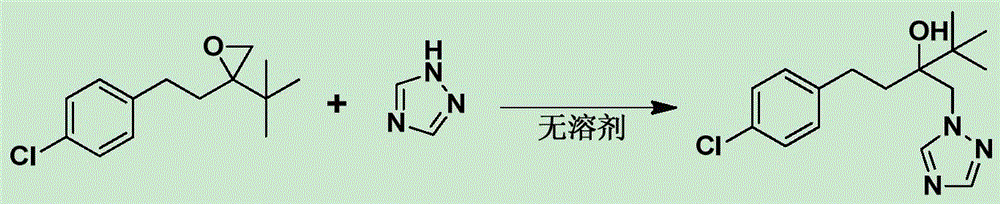
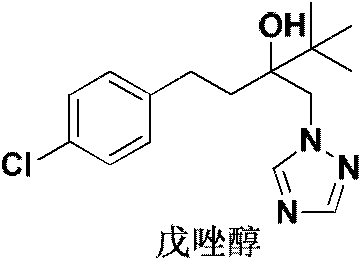
[0030] In a 250ml three-necked flask, 23.8g of 2-tert-butyl-2-(4-chlorophenethyl)oxirane was added, 10.3g of potassium carbonate and 3.6g of tetrabutylammonium bromide were added, and 1,2 , 8.2g of 4-triazole, the temperature was raised and controlled at 120°C, the reaction was carried out for 5 hours, and the reaction was stopped. Add 50g of water, add 110g of toluene, naturally cool down to 40°C and keep warm for 0.5h, then filter with suction. The filter cake was dried to obtain 26.4 g of the product tebuconazole (white solid), with a content of 98.3% and a yield of 86.1%. The chemical reaction equation of the present embodiment is as figure 1 shown.
Embodiment 2
[0032] In a 250ml three-necked flask, add 2-tert-butyl-2-(4-chlorophenethyl)oxirane 35.7g, add sodium hydroxide 3.0g and PEG 2000 12.8g, and add 1,2,4- 12.0 g of triazole was heated up and controlled at 115° C., reacted for 6 hours, and stopped the reaction. Add 42g of water, add 100g of methylcyclohexane, naturally cool down to 30°C and keep it warm for 3h, then filter with suction. The filter cake was dried to obtain 42.6 g of the product tebuconazole (white solid), with a content of 98.6% and a yield of 92.5%. The chemical reaction equation of the present embodiment is as figure 1 shown.
Embodiment 3
[0034] In a 250ml three-necked flask, add 2-tert-butyl-2-(4-chlorophenethyl)oxirane 29.8g, add triethylamine 4.4g and PEG 4000 7.3g, and add 1,2,4- 10.3 g of triazole was heated up and controlled at 125° C., reacted for 8 hours, and stopped the reaction. Add 38g of water, add 110g of cyclohexane, naturally cool down to 30°C for 2h, and filter with suction. The filter cake was dried to obtain 34.7 g of the product tebuconazole (white solid), with a content of 98.1% and a yield of 88.2%. The chemical reaction equation of the present embodiment is as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com