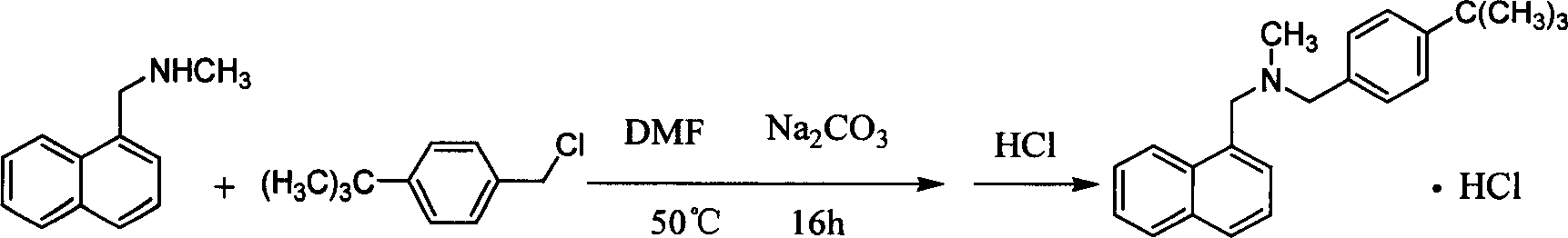Method for preparing butenafine hydrochloride
A technology of butenafine hydrochloride and acid-binding agent, which is applied in the field of medicine, can solve problems such as environmental pollution, and achieve the effects of reducing environmental pollution, reducing costs, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
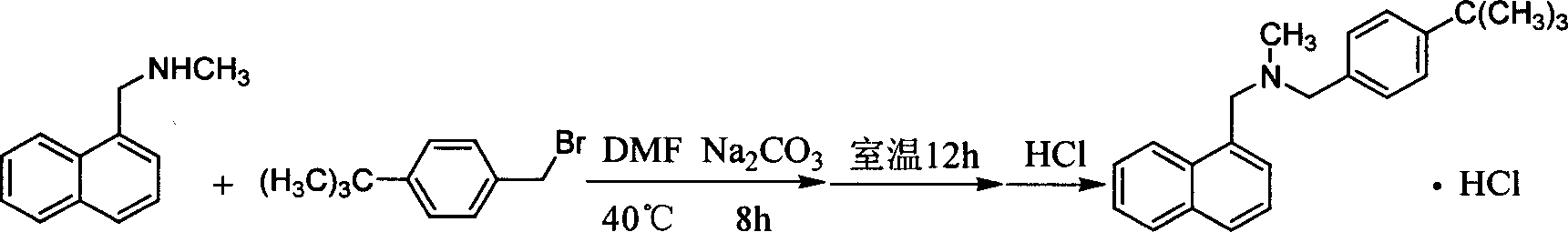
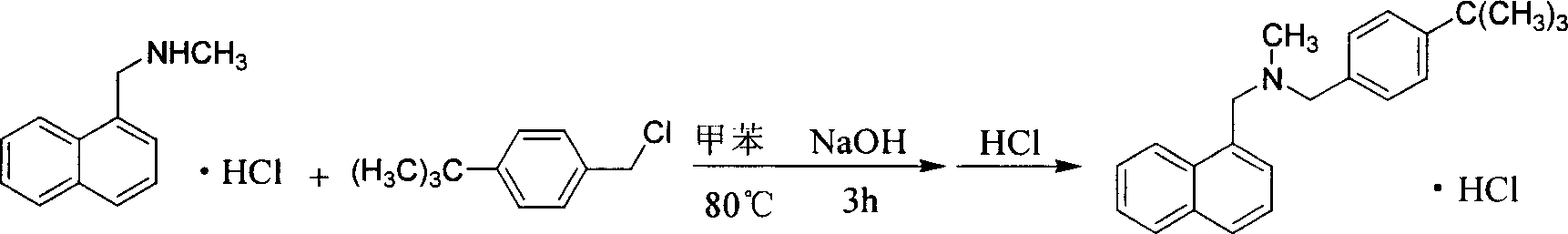
[0024] Take 77g of N-methyl-1-naphthylmethylamine, 60g of polyethylene glycol-600, and 200ml of 5N NaOH solution, heat it to 95°C, slowly add 82g of p-tert-butylbenzyl chloride dropwise, and keep it warm for 3h. Cool down, add 300ml of chloroform to extract, wash with water 3 times, add 150ml of 6N HCl, measure pH to be about 2, separate to remove hydrochloric acid, wash with water until neutral, evaporate chloroform, add 300ml of ethyl acetate to reflux, filter, and recrystallize from ethanol-acetone to obtain 134g Product, yield 86.5%, MP: 211~215°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com