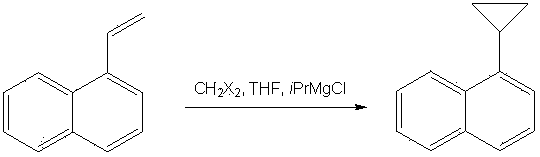Method for synthesizing 1-cyclopropyl-naphthyl by virtue of 1-vinyl-naphthyl
A technology of cyclopropyl naphthalene and vinyl naphthalene, which is applied in the direction of condensation between hydrocarbons and non-hydrocarbons to produce hydrocarbons, organic chemistry, etc., can solve the problem of high cost, and achieve the effects of simple operation, mild reaction conditions and lower synthesis costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Under nitrogen protection and ice bath conditions, 154 g (1 mol) of 1-naphthylethylene was slowly added dropwise to 100 ml of 3 M Grignard reagent tert-butylmagnesium chloride. After the dropwise addition, 187 g (1 mol) of 1,2-dibromomethane and 667 ml of 3 M Grignard reagent tert-butylmagnesium chloride were added. After reacting for 6 hours, the reaction was quenched with saturated ammonium chloride solution, extracted with ethyl acetate, and the ethyl acetate was distilled off under reduced pressure. Toluene was recrystallized to obtain 142 g of the product with a yield of 83%. Purity 99.1% (HPLC).
Embodiment 2
[0014] Under nitrogen protection and ice bath conditions, 154 g (1 mol) of 1-naphthylethylene was slowly added dropwise to 300 ml of Grignard reagent tert-butylmagnesium chloride with a concentration of 3 M. After the dropwise addition, 1043 g (6 mol) of 1,2-dibromomethane and 667 ml of 3 M Grignard reagent tert-butylmagnesium chloride were added. After reacting for 6 hours, the reaction was quenched with saturated ammonium chloride solution, extracted with ethyl acetate, and the ethyl acetate was distilled off under reduced pressure. Toluene was recrystallized to obtain 142 g of the product with a yield of 83%. Purity 99.1% (HPLC).
[0015]
Embodiment 3
[0017] Under nitrogen protection and ice bath conditions, 154 g (1 mol) of 1-naphthylethylene was slowly added dropwise to 667 ml of 3 M Grignard reagent tert-butylmagnesium chloride. After the dropwise addition, 536 g (2 mol) of 1,2-diiodomethane and 450 ml of 3 M Grignard reagent tert-butylmagnesium chloride were added. After reacting for 3 hours, the reaction was quenched with saturated ammonium chloride solution, extracted with ethyl acetate, and the ethyl acetate was distilled off under reduced pressure. Toluene was recrystallized to obtain 129 g of the product with a yield of 74%. Purity 99.3% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com