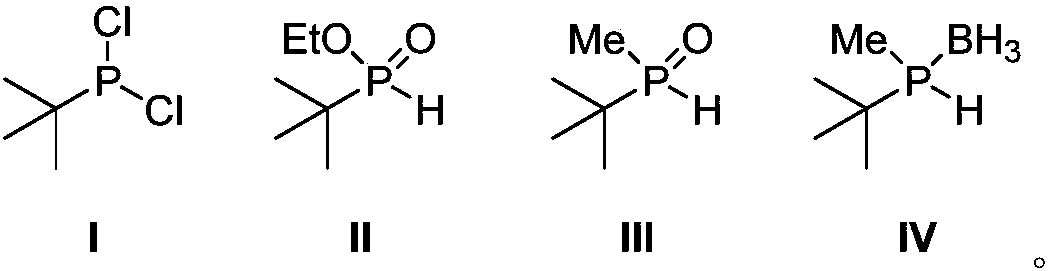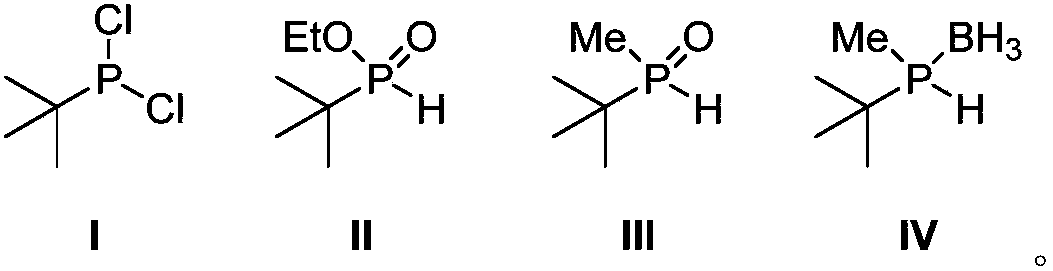Phosphorus chiral important intermediate preparation method
An intermediate and chiral technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of inability to industrially apply naphthalene lithium reagents, industrialization is difficult, and no reaction is given Steps and yields, etc., to achieve the effect of simple operation, short process route, simple reagents and reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
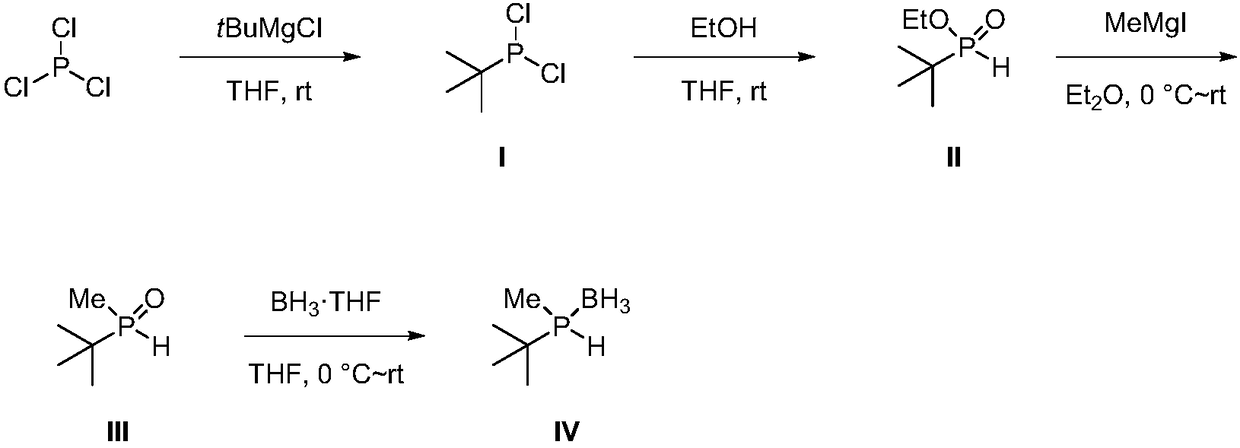
[0031] Embodiment 1, the synthesis of tert-butylphosphine dichloride (I)
[0032] In a dry 100mL single-necked flask, slowly dropwise add phosphorus trichloride (40mL, 0.46mol, 2.5eq) to a solution of tert-butylmagnesium chloride in tetrahydrofuran (1.0M, 180mL, 0.18mol), and react at room temperature for 24 hours , and filtered under nitrogen protection to obtain the target product as a white solid. The yield was 51%.
[0033] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) = 1.23 (d, J PH =14.8Hz,9H,C(CH 3 ) 3 ); 31 P NMR (162MHz, CDCl 3 ): δ (ppm) = 200.0 (s); 13 C NMR (100MHz, CDCl 3 ): δ (ppm) = 24.4 (CH 3 ),23.8(d,J PC =20.9Hz, C(CH 3 ) 3 ).
Embodiment 2
[0034] Embodiment 2, the synthesis of ethyl tert-butylphosphonite (II)
[0035] In a dry 250mL one-necked flask, a tetrahydrofuran solution (75mL) of tert-butylphosphine dichloride (10.8g, 67.9mmol) was added, and absolute ethanol (25mL, 427.9mmol, 6.3eq) was added dropwise. After reacting at room temperature for 12 hours, distillation gave the target product as a colorless oil with a yield of 84%.
[0036] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) = 6.71 (d, J PH =514.4Hz,1H,PH),4.24-4.02(m,2H,OCH 2 ),1.34(t,J HH =7.2Hz,3H,CH2 CH 3 ),1.11(t,J PH =17.6Hz,9H,C(CH 3 ) 3 ).
Embodiment 3
[0037] Embodiment 3, the synthesis of ethyl tert-butylphosphonite (II)
[0038] A toluene solution (20 mL) of tert-butylphosphine dichloride (5.4 g, 34.0 mmol) was added to a dry 150 mL one-necked bottle, and absolute ethanol (15 mL, 256.7 mmol, 7.5 eq) was added dropwise. After reacting at 0° C. for 24 hours, distillation gave the target product as a colorless oil with a yield of 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com