Chemical method used for preparing aromatic cyclopropanecarbonitrile and cyclopropylamine
An aryl and system technology, applied in chemical instruments and methods, nitrile preparation, organic chemistry and other directions, can solve the problems of high cost and low product yield, and achieve the effects of easy handling, high stereoselectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation method of compound (2S)-2-(3,4-difluorophenyl)oxirane of formula (V-1)
[0065]
[0066] (V-1)
[0067] Combine (1S)-2chloro-1-(3,4-difluorophenyl)-1-ethanol (50g, prepared according to the method disclosed in CN10149544), toluene (128ml), sodium hydroxide (11g) and water (106ml) ) were mixed, the reaction was stirred at 40° C. for 1 hour, the organic solvent was separated, washed with water, and the solvent was evaporated under reduced pressure to obtain 38.16 g of the compound of formula (V-1) (yield: 94%).
[0068] 1 H-NMR: (CDCl 3 ): δ 2.71-2.73 (1H, dd), 3.13-3.15 (1H, m), 3.82-3.83 (1H, m), 7.01-7.27 (4H, m).
Embodiment 2
[0070] Preparation method of compound (1R, 2R)-2-(3,4-difluorophenyl)cyclopropanenitrile of formula (IV-1)
[0071]
[0072] (IV-1).
[0073] NaH (45g, 60%, 1.13mol) and ethylene glycol dimethyl ether (300ml) were combined and stirred. To the resulting suspension was added dropwise a solution of diethyl cyanomethyl phosphate (166.4 g, 0.94 mol) in ethylene glycol dimethyl ether (120 ml) at 0°C. A solution of (2S)-2-(3,4-difluorophenyl)oxirane (38g, 244mmol) in ethylene glycol dimethyl ether (200ml) was slowly added dropwise at 40-60°C. , the reaction was carried out at 60°C, monitored by TLC, and the reaction was completed in about 10 hours. Cooled to room temperature, separated the organic layer, washed with water, concentrated, and precipitated 40.5 g of solid, the yield was 92.8%, the HPLC purity was >98%, and the ee value was greater than 99.9%.
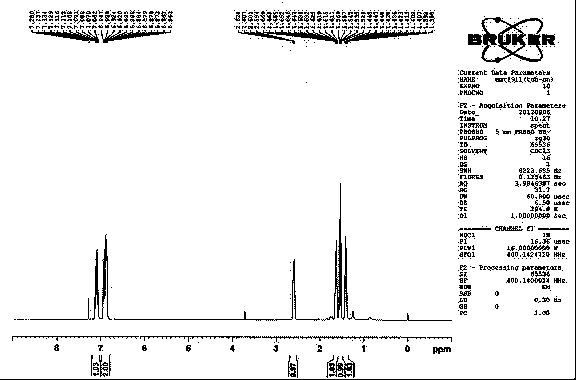
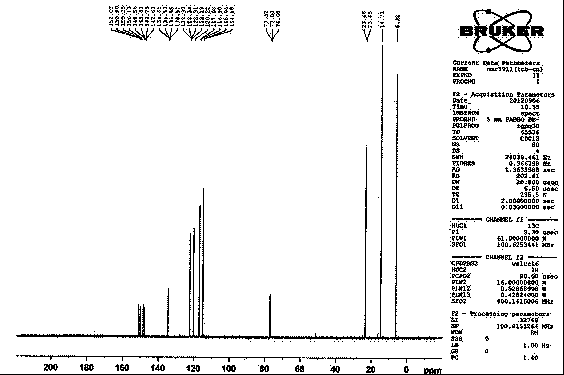
[0074] of the compound 1 H-NMR and 13 C-NMR as figure 1 and figure 2 shown.
Embodiment 3
[0076] Preparation method of compound (1R, 2R)-2-(3,4-difluorophenyl)cyclopropylcarboxylic acid
[0077]
[0078] (1R,2R)-2-(3,4-difluorophenyl)cyclopropanenitrile (20g) prepared in Example 2 and aqueous sodium hydroxide solution (10%, 100ml) were heated and refluxed for about 4 hours to The reaction is complete. Cool to room temperature, concentrate, and extract with ether. It was acidified by dropwise addition of 15% HCl, extracted with ether, and concentrated to obtain 21.0 g of (1R,2R)-2-(3,4-difluorophenyl)cyclopropylcarboxylic acid with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com