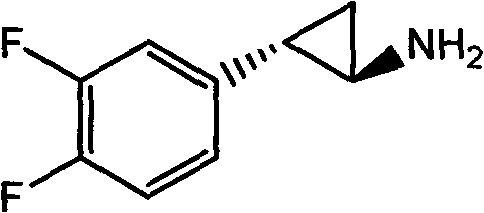
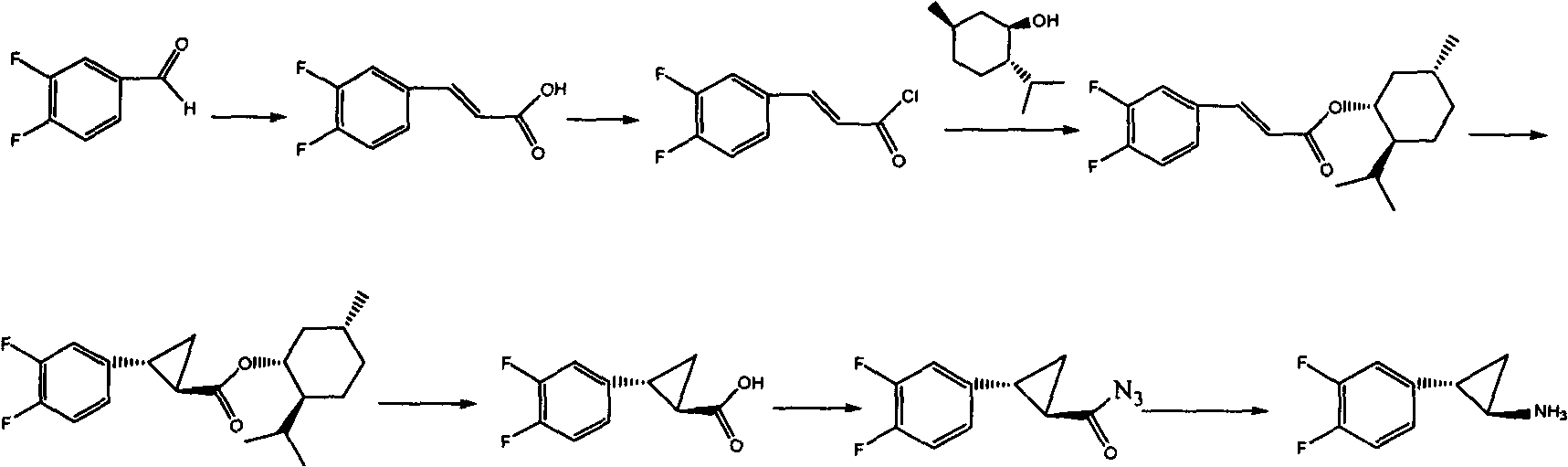
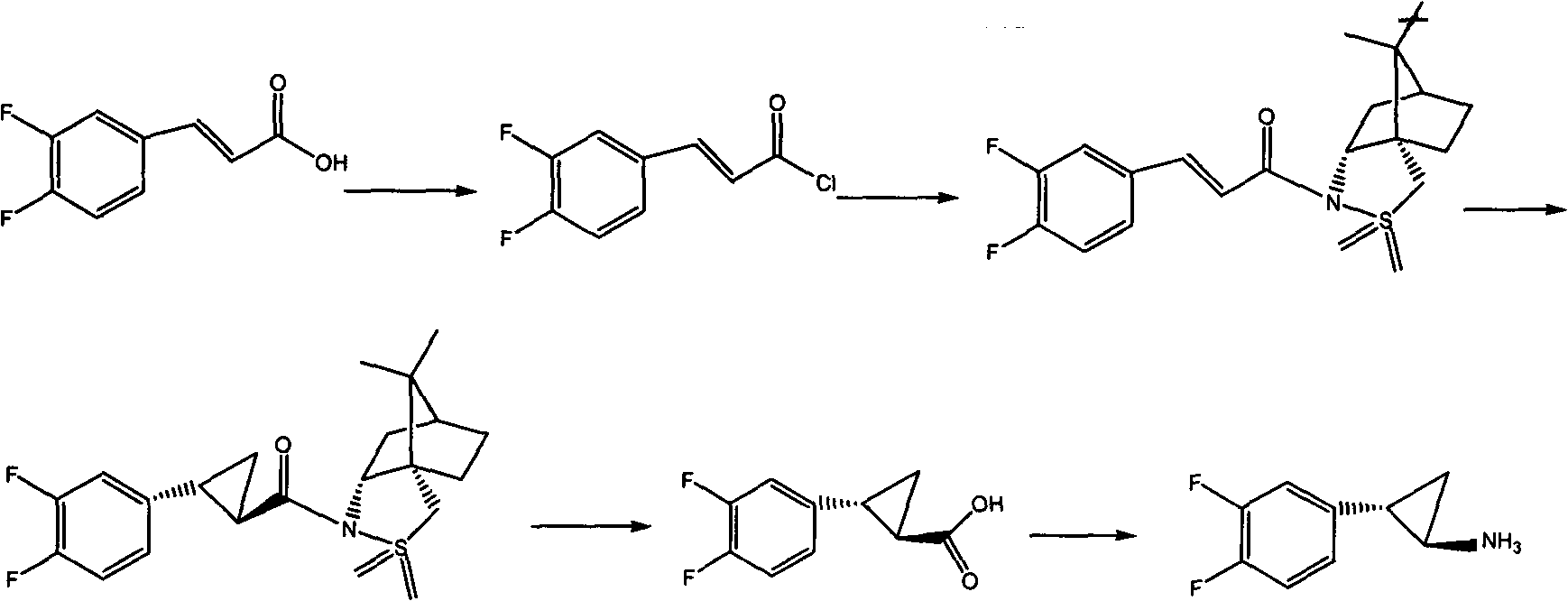
Preparation method of Ticagrelor intermediate
A technology for ticagrelor and intermediates, which is applied in the field of preparation of ticagrelor intermediates, can solve the problems of unsuitability for large-scale industrial production, column purification of products, and many side reactions, and achieve high product yields and excellent reaction conditions. Gentle and easy to control, easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] The present invention is further illustrated by the following examples, which are only for illustration and not for limiting the present invention.
[0052] The preparation method of this ticagrelor intermediate adopts the following steps:
[0053] (1) Preparation of ethyl 3,4-difluorocinnamate:
[0054] a) Preparation of phosphorus ylide feed liquid:
[0055] Add 16.6g of triethyl phosphite, 80ml of tetrahydrofuran, and 12.2ml of ethyl α-bromoacetate to the first reaction flask in sequence, stir, heat, and reflux for 2 hours, stop heating, cool down to room temperature naturally, and then control the temperature in an ice-water bath Add 12.5 g of sodium tert-butoxide in batches to the first reaction flask at around 0°C, stir for 1 hour, and use it directly for the reaction of preparing ethyl 3,4-difluorocinnamate;
[0056] b) Preparation of ethyl 3,4-difluorocinnamate:
[0057] Add 12.8g of 3,4-difluorobenzaldehyde (I) and 60ml of tetrahydrofuran to the second react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com