Method for preparing ticagrelor key intermediate and racemate thereof and special intermediate for implementing method
A technology of ticagrelor and racemate, which is applied in the new synthesis field of key intermediates of ticagrelor, can solve the problems of high cost of camphorsulfonamide, poor ring-closing selectivity, and low total yield, and achieves The effect of simple and convenient post-processing, environmental friendliness, safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070]
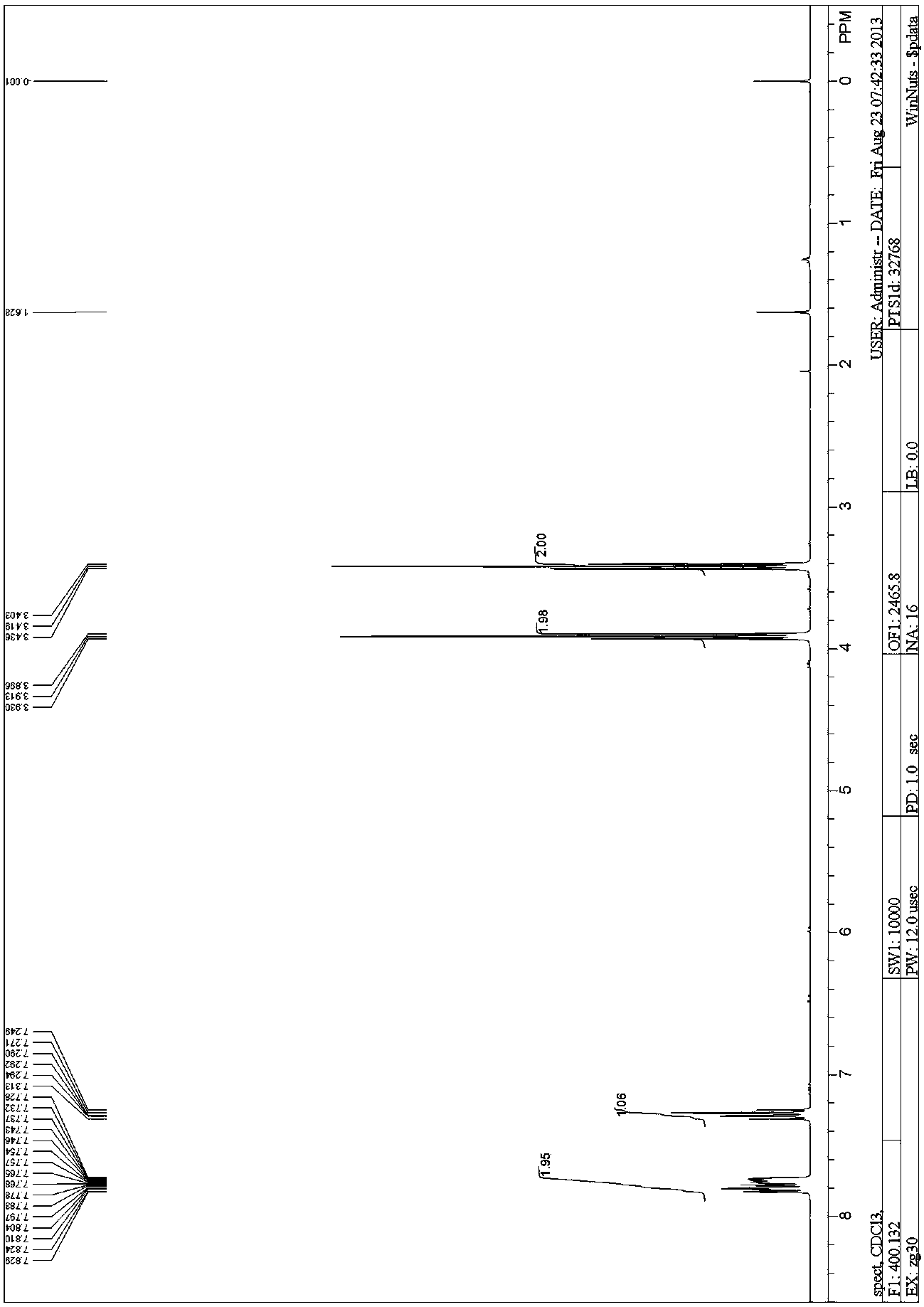
[0071] At an internal temperature lower than 30°C, 1,2-difluorobenzene (20g, 0.175mol, 1eq) and A mixture of 3-chloropropionyl chloride (24.30g, 0.193mol, 1.1eq). Keep the temperature of the reaction system below 30°C. After the dropwise addition, the reaction was stirred overnight at room temperature, and the reaction progress was monitored by TLC. After the reaction was complete, the reaction mixture was slowly poured into ice water, the organic phase was separated, washed with water, dried and concentrated to obtain the crude yellow liquid compound I. No purification is required. Yield: 31.5 g, yield: 88%.
[0072] 1 HNMR (400MHz, DMSO- d 6 ): δ7.829-7.728(m, 2H), 7.294-7.249(m, 1H), 3.913(t, J =6.8 Hz, 2H), 3.419(t, J =6.8 Hz, 2H).
Embodiment 2
[0074]
[0075] 1) Under stirring at room temperature, trimethyl borate (1.67g) was added to a mixture of L-diphenylprolinol (2.9g) in toluene (75mL). After the mixture was stirred at 40°C for 1 hour, borane dimethyl sulfide (10mol / L, 13.9g) was added dropwise, and the temperature was controlled not to be higher than 45°C. The mixture was stirred at 40°C for 1 hour.
[0076] 2) Slowly add a solution of 3-chloro-1-(3,4-difluorophenyl)propyl-1-one (compound I, 46.8g) in toluene (110mL) to the above mixture, and control the temperature at 35°C-40°C. After the dropwise addition, the reaction system was stirred at 40° C. for 1 hour. TLC (PE / EA, 3 / 1, V / V) followed the completion of the reaction. The temperature of the reaction system was lowered to 10°C, methanol (40 mL) was slowly added to quench the reaction, the temperature was controlled below 35°C, and stirred for 30 minutes. The mixture was concentrated to about 100 mL under reduced pressure, and washed three times with...
Embodiment 3
[0079]
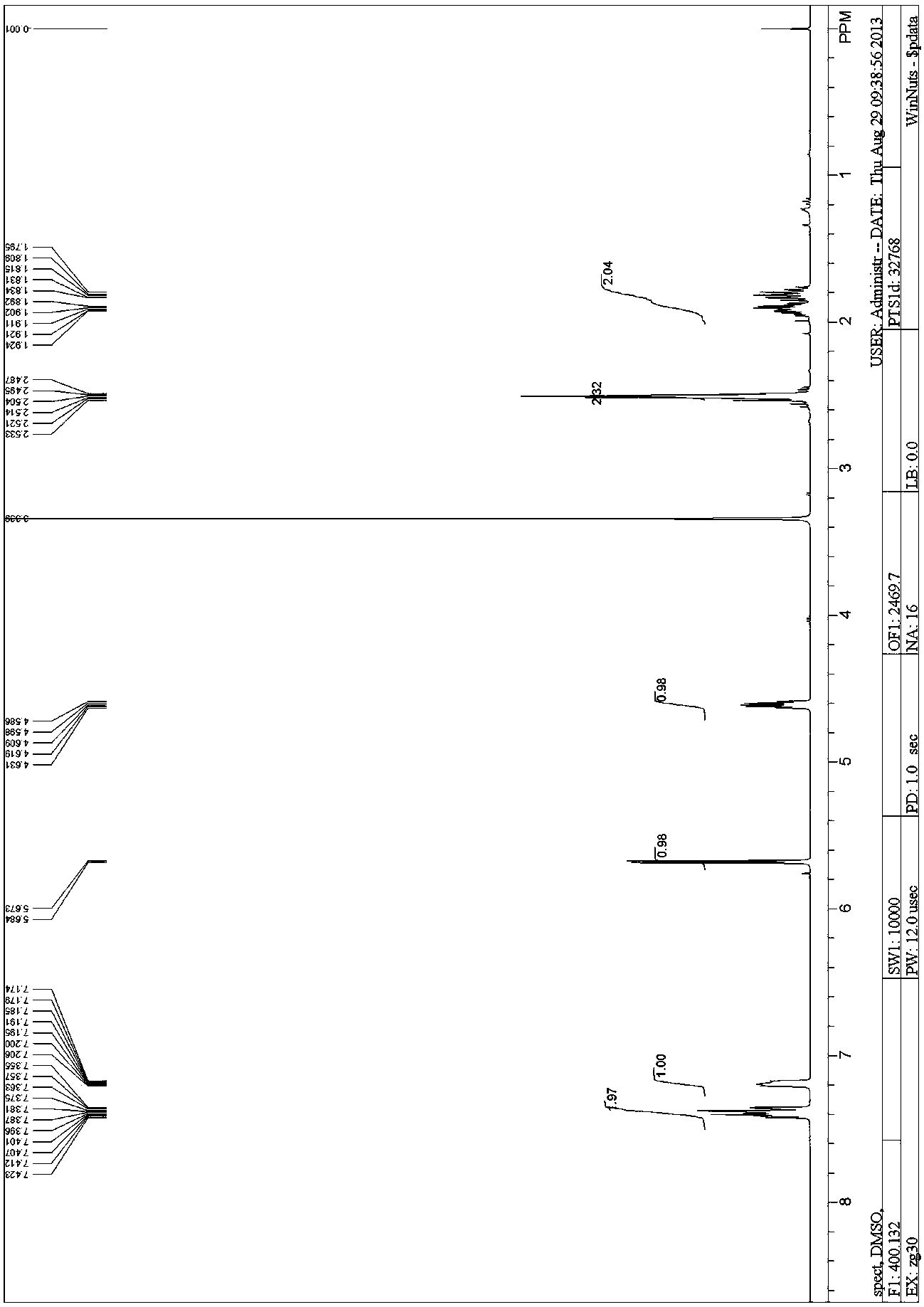
[0080] Compound II (6.2 g, 3 mmol, 1 eq) was dissolved in dimethyl sulfoxide (20 mL) at room temperature. Sodium cyanide (2.14g, 4.5mol, 1.5eq) was added carefully. The reaction was stirred overnight at 60 °C. After TLC showed that there was no raw material, cool, add 50 mL of water, extract with ethyl acetate (100 mL), concentrate the organic phase, add 50 mL of water to the residue, extract with ethyl acetate (100 mL), dry the organic phase with anhydrous sodium sulfate, and concentrate. The crude product of compound III (6.3 g) was obtained, which was directly put into the next reaction.
[0081] 1 HNMR (400MHz, DMSO- d 6 ): δ1.795-1.834(m, 1H), 1.892-1.924(m, 1H), 2.487-2.533(m, 2H), 4.586-4.631(m, 1H), 5.679(d, J =4.4 Hz, 1H), 7.174-7.206(m, 1H),7.355-7.423(m, 2H).
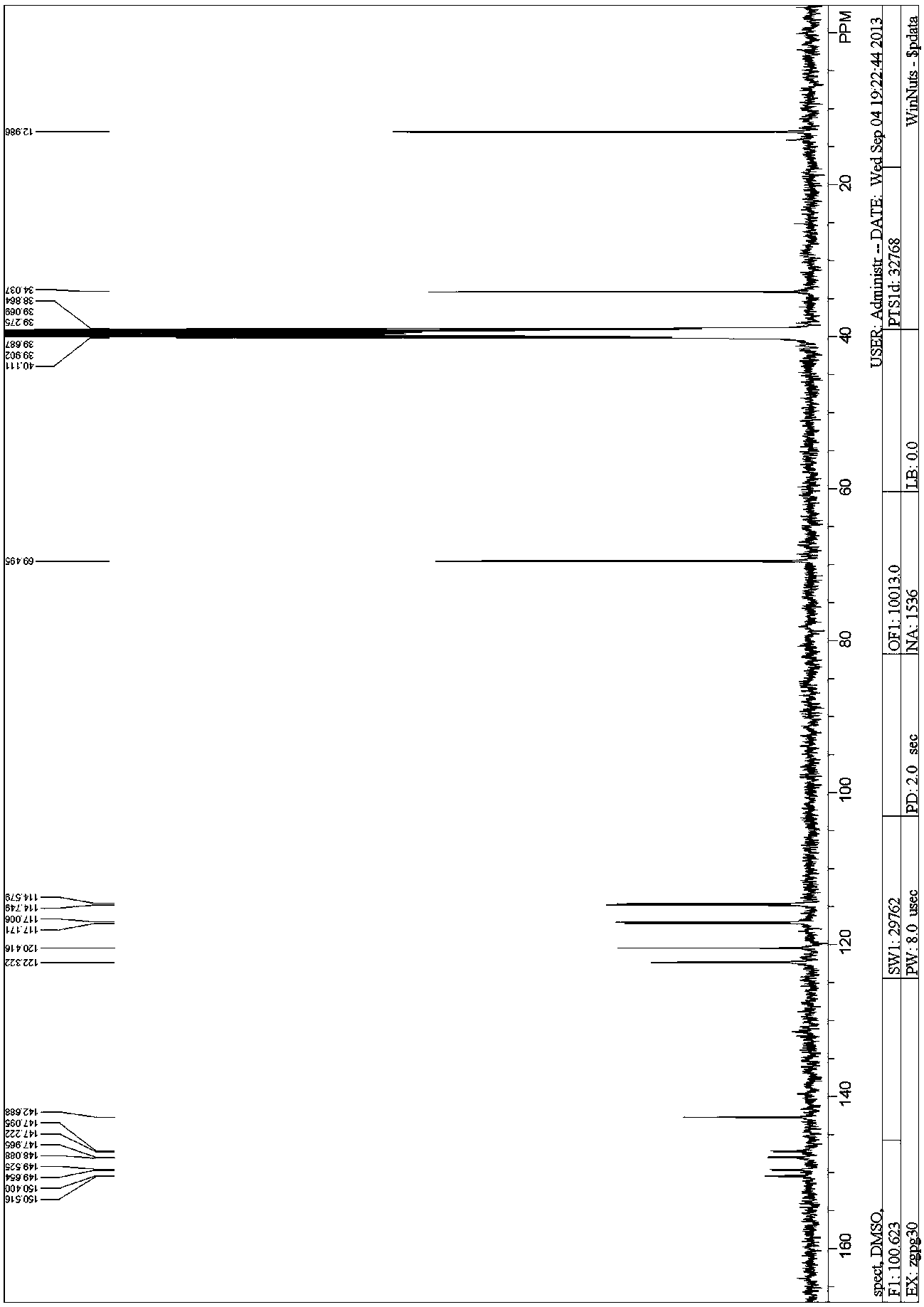
[0082] 13 CNMR (100MHz, DMSO- d 6 ): δ12.986, 34.037, 69.495, 114.664(d, J =17 Hz), 117.088(d, J =17 Hz), 120.416, 122.322, 142.688, 147.655(dd, J 1 =86.6Hz, J 2 =12.5 Hz), 150.085(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com