Tech. of preparing 3,3-dichlorobenzidine hydrochloride
A dichlorobenzidine hydrochloride and process technology, applied in the chemical industry, can solve the problems of selectivity of transposition products, uneven mixing, and complicated processes that affect the conversion rate of transposition, achieve low cost, reduce heat transfer, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
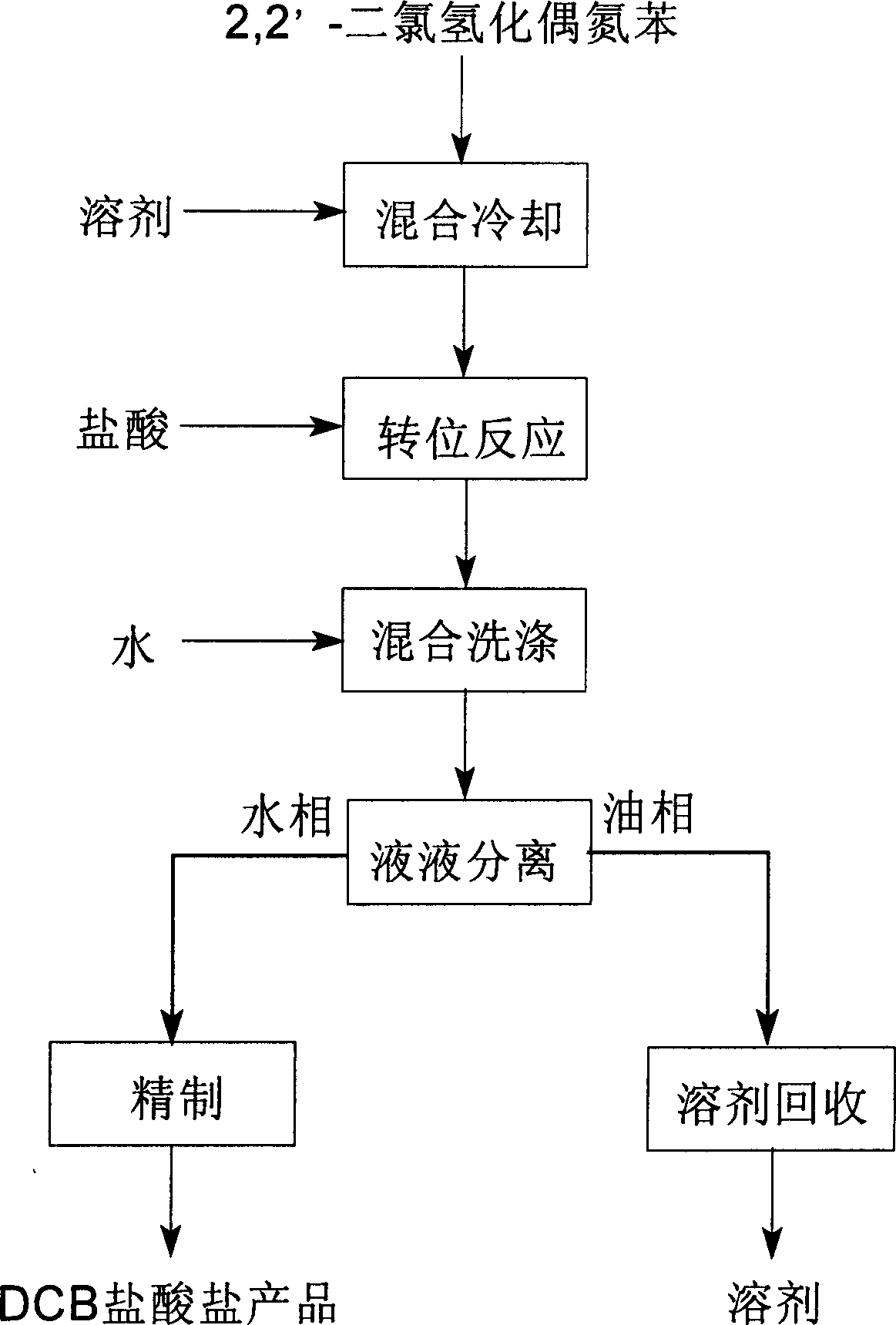
[0025] A kind of preparation 3,3'-dichlorobenzidine hydrochloride technique, described process step is as follows:
[0026] (1). Mixing and cooling, the aromatic hydrocarbon toluene solvent of 2,2'-dichlorohydroazobenzene is mixed into 2,2'-dichlorohydroazobenzene toluene solution, then cooled to 10°C±2°C.
[0027] (2). Transposition reaction, 2,2'-dichlorohydroazobenzenetoluene solution is slowly all added dropwise to 1000 liters of hydrochloric acid solution in the cooled propulsion type transposition reactor to carry out transposition reaction, 2, The volume ratio of 2'-dichlorohydroazobenzene to the added hydrochloric acid solution is 1:1.2, the concentration of the added hydrochloric acid solution is 35%, the temperature is 10°C±2°C, and the temperature is kept at 10°C during the transposition reaction ±5°C, continue to stir for 1.5h, keep the temperature at 10±5°C, make the transposition reaction between 2,2'-dichlorohydroazobenzene and hydrochloric acid, the total opera...
Embodiment 2
[0032] A kind of preparation 3,3'-dichlorobenzidine hydrochloride technique, described process step is as follows:
[0033] (1). Mixing and cooling, the aromatic hydrocarbon toluene solvent of 2,2'-dichlorohydroazobenzene is mixed into 2,2'-dichlorohydroazobenzene toluene solution, then cooled to 10°C±2°C.
[0034] (2).Transposition reaction, 2,2'-dichlorohydroazobenzenetoluene solution is slowly added dropwise to 800 liters of hydrochloric acid solution in the cooled propulsion type transposition reactor, and the temperature is maintained in the process of adding. 10°C±5°C, after adding 2,2'-dichlorohydroazobenzene toluene solution, continue to add 200 liters of hydrochloric acid to the reaction system and continue stirring for 1.5h, 2,2'-dichlorohydroazobenzene and hydrochloric acid The volume ratio of the solution is 1: 1.2, the concentration of the added hydrochloric acid solution is 35%, and the temperature is kept at 10 ± 5°C to make 2,2'-dichlorohydroazobenzene and hydr...
Embodiment 3
[0039] A kind of preparation 3,3'-dichlorobenzidine hydrochloride technique, described process step is as follows:
[0040] (1). Mixing and cooling, the aromatic hydrocarbon toluene solvent of 2,2'-dichlorohydroazobenzene is mixed into 2,2'-dichlorohydroazobenzene toluene solution, then cooled to 10°C±2°C.
[0041] (2).Transposition reaction, 2,2'-dichlorohydroazobenzenetoluene solution is slowly added dropwise to 640 liters of hydrochloric acid solution in the cooled propulsion type transposition reactor, and the temperature is maintained in the process of adding. 10°C±5°C, after adding 2,2'-dichlorohydroazobenzene toluene solution, continue to add 200 liters of hydrochloric acid to the reaction system and continue stirring for 1.5h, 2,2'-dichlorohydroazobenzene and hydrochloric acid The volume ratio of the solution is 1: 1.0, the concentration of the added hydrochloric acid solution is 35%, and the temperature is maintained at 10±5° C., so that 2,2'-dichlorohydroazobenzene a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com