Preparation method for pentafluorophenol
A technology of pentafluorophenol and equation, applied in the field of preparation of pentafluorophenol, can solve the problems of high cost of raw materials, long process route, high production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
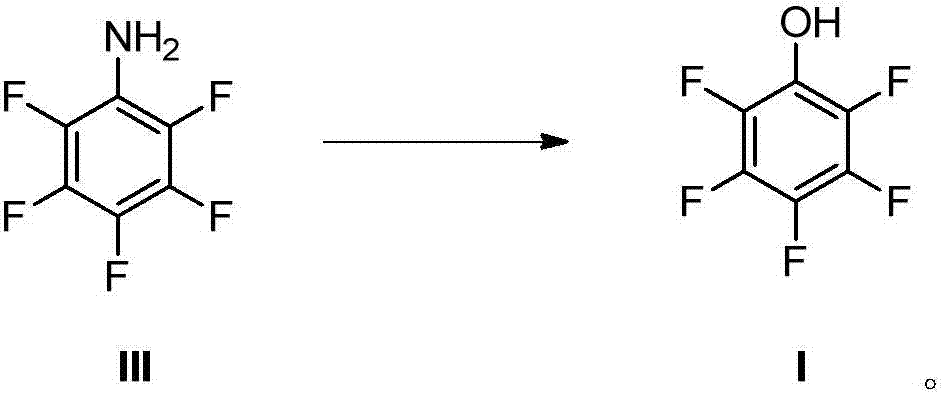
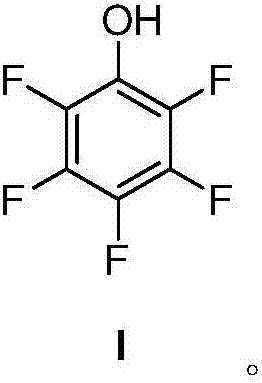
[0065] The present invention provides a kind of preparation method of pentafluorophenol, the structure of described pentafluorophenol is as shown in formula I:
[0066]
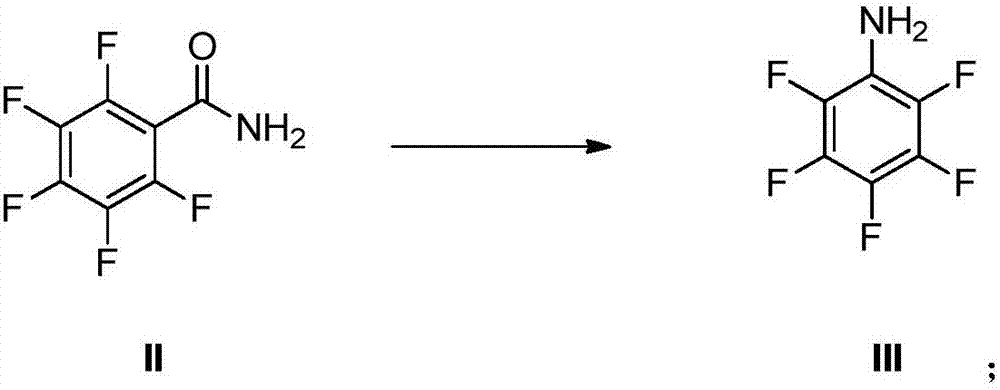
[0067] The preparation method of pentafluorophenol provided by the present invention may comprise Hoffman rearrangement reaction, and described Hoffman rearrangement reaction specifically comprises: under the condition that the compound of formula II is carried out Hoffman rearrangement under the condition that alkali, halogenating reagent exist Exhaust reaction, preparation obtains formula III compound, and reaction equation is as follows:
[0068]
[0069] In the preparation method of pentafluorophenol provided by the present invention, the Hofmann rearrangement reaction can be carried out in a reaction solvent, and those skilled in the art can make the reaction according to the reaction raw materials (for example, formula II compound, alkali, halogenating reagent, etc.) Select the appropriate solvent...
Embodiment 1
[0086] Add 68g of sodium hydroxide to 1200g of water at 0-10°C. Add 75g of bromine dropwise to aqueous sodium hydroxide solution, and stir at 0-10°C for 1h. 90g of pentafluorobenzamide solid was added in batches to the above reaction solution, and the internal temperature was controlled to 80°C for reaction. After the reaction was completed, the temperature was lowered to 20°C, 200g of methyl tert-butyl ether was added to the reaction liquid, stirred, left standing, and separated into layers, and the organic phase was concentrated at 30°C to obtain 69g of pentafluoroaniline with a yield of 89.5%, which was analyzed by HPLC , content >95%.
Embodiment 2-6
[0088] The kind of organic solvent is changed, and other is with embodiment 1, and its result is shown in Table 1:
[0089] Table 1
[0090]
[0091]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com