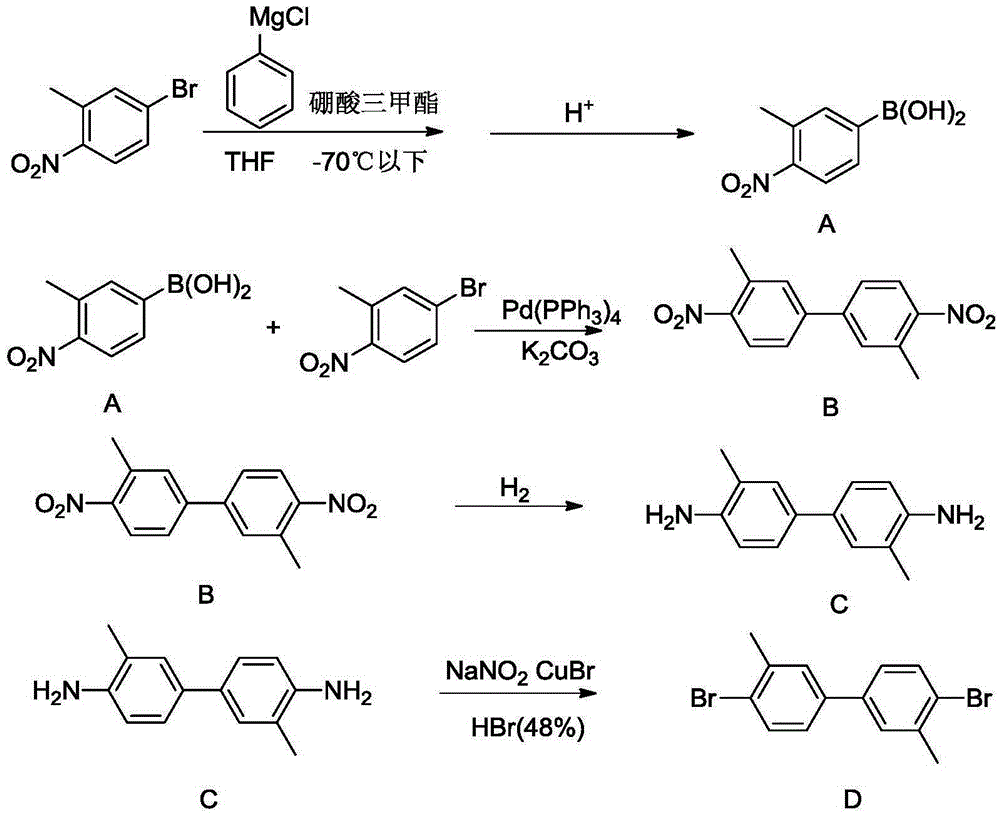
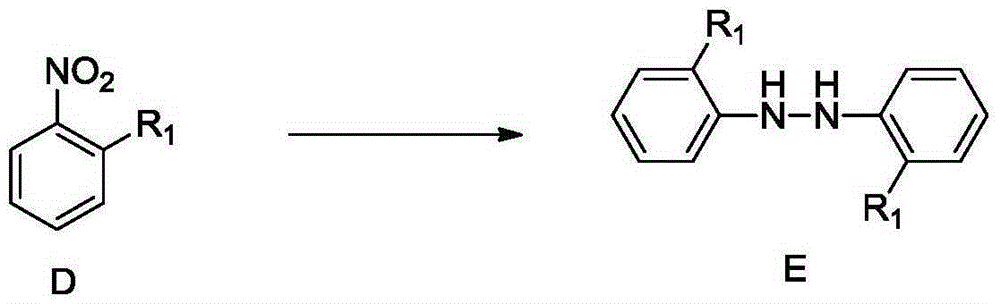
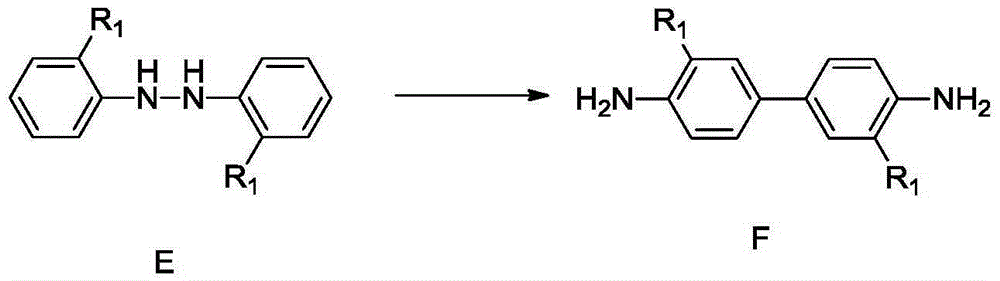
Synthetic method for 4,4'-dihalogenated-3,3'-dialkyl(alkoxyl) biphenyl compounds
A synthesis method and compound technology are applied in the field of synthesis of 4,4'-dihalogen substituted-3,3'-dialkylbiphenyl compounds, which can solve difficult scale production, harsh conditions, high price, etc. problem, to achieve the effect of mild reaction conditions, simple post-processing and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0035] Example 14, Synthesis of 4'-dibromo(chloro / iodine)-3,3'-dimethylbiphenyl (G1-A~G1-C)
[0036]
[0037] Synthesis of 2,2'-dimethylhydroazobenzene (E1):
[0038] Dissolve 102.8g (0.75mol) of the raw material o-nitrotoluene in 700g of ethanol with a mass concentration of 95%, pour it into an autoclave, add 1.5g of 1,4-naphthoquinone, and then add 700g of a 50% mass concentration of sodium hydroxide solution , 1.5g sodium dodecylbenzenesulfonate and 1.5g Pd / C catalyst containing Pd0.8wt%, after replacing the air in the autoclave three times with nitrogen, then replace it with hydrogen three times, seal the autoclave, start stirring, and heat to React under the conditions of 30°C and 0.6MPa until the pressure of the autoclave no longer changes, cool down to 20-25°C, discharge the material, filter out the Pd / C catalyst and reuse it. The filtrate was separated into layers, the organic layer was taken, and the solvent was evaporated to dryness by rotary evaporation to obtai...
Embodiment 2
[0050] Example 2: Synthesis of 4,4'-dibromo(chloro / iodine)-3,3'-dimethoxybiphenyl (G2-A~G2-C)
[0051]
[0052] Synthesis of 2,2'-dimethoxyhydroazobenzene (E2):
[0053] Dissolve 114.8g (0.75mol) of the raw material o-nitromethoxybenzene in 600g of ethanol with a mass concentration of 95%, pour it into an autoclave, add 1.5g of 1,4-naphthoquinone, and then add 600g of hydrogen with a mass concentration of 50% Sodium oxide solution, 1.72g sodium dodecylbenzene sulfonate and 1.72g Pd / C catalyst containing Pd0.8wt%, nitrogen replacement autoclave inner air three times, then use hydrogen replacement three times, autoclave is sealed, start stirring, Heating to 35°C and 0.6MPa to react until the pressure of the autoclave no longer changes, then cool down to 20-25°C, discharge the material, filter out the Pd / C catalyst, and reuse it. The filtrate was separated into layers, the organic layer was taken, and the solvent was evaporated by rotary evaporation to obtain 85.4 g of solid ...
Embodiment 3
[0065] Example 3: Synthesis of 4,4'-diiodo(chloro / bromo)-3,3'-diethoxybiphenyl (G3A-G3C)
[0066]
[0067] Synthesis of 2,2'-diethoxyhydroazobenzene (E3):
[0068] Dissolve 125.7 g (0.75 mol) of raw material D3 o-nitroethoxybenzene in 817.05 g of ethanol with a mass concentration of 95%, pour it into an autoclave, add 1.5 g of 1,4-naphthoquinone, and then add 817.05 g of a mass concentration of 50 % sodium hydroxide solution, 1.9g sodium dodecylbenzenesulfonate and 1.9g containing the Pd / C catalyst of Pd0.8wt%, nitrogen replacement air three times, and then use hydrogen replacement three times, autoclave is sealed, open and stir, Heating to 35°C and 0.6MPa to react until the pressure of the autoclave no longer changes, then cooling down to 20-25°C, discharging, and filtering out the Pd / C catalyst for reuse. The filtrate was separated, the organic layer was taken, and the solvent was evaporated to dryness by rotary evaporation to obtain 96 g of solid 2,2'-diethoxyhydroazobe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com