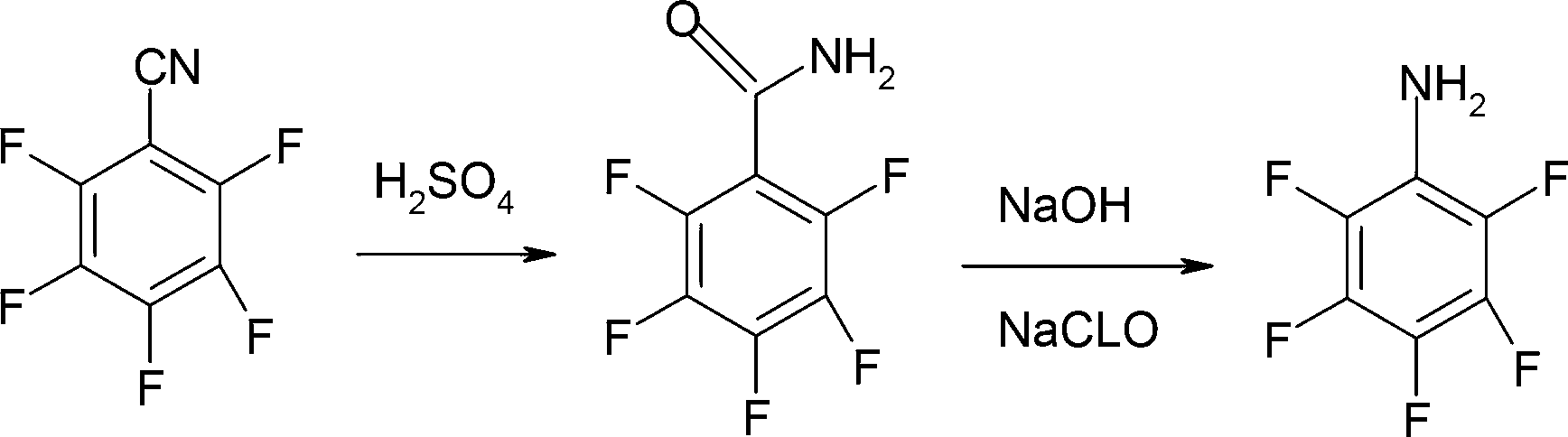
Preparation method of pentafluoroaniline
A technology of pentafluoroaniline and pentafluorobenzamide, which is applied in the chemical industry, can solve the problems of unsuitability for industrial production, harsh reaction conditions, and difficulty in obtaining hexachlorobenzene, and achieve the effects of low cost, convenient operation, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step (1): In a 1000ml four-neck flask, start stirring, add 120ml of water and 800g (8.00mol) of 98% sulfuric acid in sequence, then add 240g (1.24mol) of pentafluorobenzonitrile, heat up to 110°C for 4 hours, Then the reaction solution was added to water for crystallization, stirred at room temperature for 30 minutes, filtered, the filter cake was washed with water until neutral, and dried to obtain 249 g of pentafluorobenzamide, the gas phase content was ≥99%, and the yield was 94.9%.
[0020] Step (2a): Under stirring, add 500ml of water into a 2000ml four-necked flask, and add 90g (0.43mol) of pentafluorobenzamide obtained in step (1), and then cool the suspension to 0-10°C. Then, add 60ml (0.45mol) of 30% sodium hydroxide solution under stirring, and keep the temperature within the range of 0-10°C while adding. Next, 300 g (0.48 mol) of 12% sodium hypochlorite solution was added under stirring. During the entire dropping period, the temperature was kept within the r...
Embodiment 2
[0023] Step (1): In a 1000ml four-neck flask, start stirring, add 80ml of water and 800g (8.00mol) of 98% sulfuric acid in sequence, then add 240g (1.24mol) of pentafluorobenzonitrile, heat up to 100°C for 4 hours, Then the reaction solution was added to water for crystallization, stirred at room temperature for 30 minutes, filtered, and the filter cake was washed with water until neutral, and dried to obtain 252 g of pentafluorobenzamide, the gas phase content was ≥99%, and the yield was 96.0%.
[0024] Step (2a): Under stirring, add 500ml of water into a 2000ml four-necked flask, and add 90g (0.43mol) of pentafluorobenzamide obtained in step (1), and then cool the suspension to 10-20°C. Then, add 60ml (0.45mol) of 30% sodium hydroxide solution under stirring, and keep the temperature within the range of 10-20°C while adding. Next, 350 g (0.57 mol) of 12% sodium hypochlorite solution was added under stirring. During the entire dropping period, the temperature was kept within ...
Embodiment 3
[0027] Step (1): In a 1000ml four-neck flask, start stirring, add 80ml of water and 800g (8.00mol) of 98% sulfuric acid in sequence, then add 240g (1.24mol) of pentafluorobenzonitrile, heat up to 90°C for 4 hours, Then the reaction solution was added to water for crystallization, stirred at room temperature for 30 minutes, filtered, the filter cake was washed with water until neutral, and dried to obtain 242 g of pentafluorobenzamide, the gas phase content was ≥99%, and the yield was 92.2%.
[0028] Step (2a): Under stirring, add 500ml of water into a 2000ml four-necked flask, and add 90g (0.43mol) of pentafluorobenzamide obtained in step (1), and then cool the suspension to 0-10°C. Then, add 60ml (0.45mol) of 30% sodium hydroxide solution under stirring, and keep the temperature within the range of -10~0°C while adding. Next, 350 g (0.57 mol) of 12% sodium hypochlorite solution was added under stirring. During the entire dropping period, the temperature was kept in the range ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com