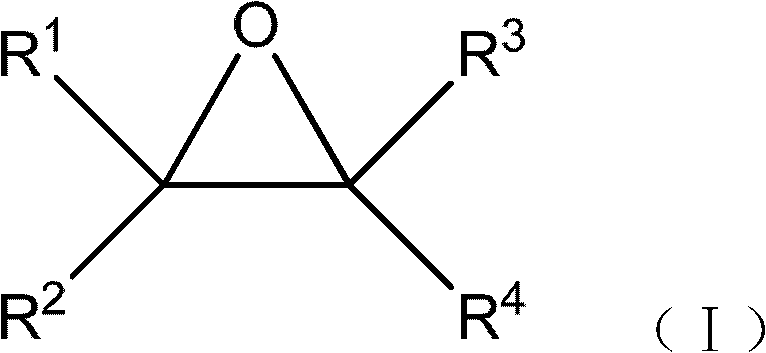
Preparation methods of 2,2-dimethyl-1,3-dioxolane and ethylene glycol
A technology of dioxolane and dimethyl, which is applied in the field of preparing ethylene glycol, can solve problems such as the high price of zinc trifluoromethanesulfonate and environmental pollution, and is beneficial to recycling, improving catalytic efficiency, and overcoming expensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0022] According to a preferred embodiment of the present invention, the cation of the ionic liquid 1-alkyl-3-methylimidazolium salt is 1-butyl-3-methylimidazolium, and the anion is bromide, chloride, trifluoro One of methanesulfonate ion, methanesulfonate ion, p-toluenesulfonate ion.
[0023] Specifically, the ionic liquid 1-alkyl-3-methylimidazolium salt can be 1-butyl-3-methylimidazole bromide, 1-butyl-3-methylimidazole chloride, 1-butyl -3-methylimidazolium bisulfate, 1-butyl-3-methylimidazolium methanesulfonate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate, 1-butyl-3-methyl imidazole p-toluenesulfonate, etc., more preferably the ionic liquid is 1-butyl-3-methylimidazole bromide. The ionic liquids mentioned above can be prepared by methods known in the art (see "Green Reaction Media in Organic Synthesis" edited by Koichi Mikami, 2005, Blackwell Publishing Ltd. et al.).
[0024] According to a preferred embodiment of the present invention, the ionic liquid 1-alky...
Embodiment 1
[0045] Put the magnetic stirring bar into a 25mL polytetrafluoroethylene-lined reaction kettle, add 0.1mol (5.8g) acetone, 0.025mol (1.1g) ethylene oxide, and then add 0.01mol 1-butyl bromide- 3-Methylimidazolium ionic liquid and 0.005mol (0.68g) of anhydrous zinc chloride are uniformly mixed as a catalyst, and the reaction kettle is closed, and stirred and reacted for 8 hours in an oil bath at 65°C. After the reaction was completed and cooled to room temperature, a liquid sample was taken for analysis. The conversion rate of ethylene oxide was 99%, and the selectivity of 2,2-dimethyl-1,3-dioxolane was 100%.
Embodiment 2
[0052] The reaction mixture obtained in Example 1 was distilled under the protection of nitrogen. After the product and the raw material were completely separated by distillation, the rest was the ionic liquid containing zinc chloride. Prepare 2,2-dimethyl-1,3-dioxolane according to the method of Example 1, and replace the catalyst of Example 1 with the recovered ionic liquid containing zinc chloride to prepare 2,2-dimethyl-1,3-dioxolane 1,3-dioxolane. The conversion of ethylene oxide was 99%, and the selectivity to 2,2-dimethyl-1,3-dioxolane was 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com