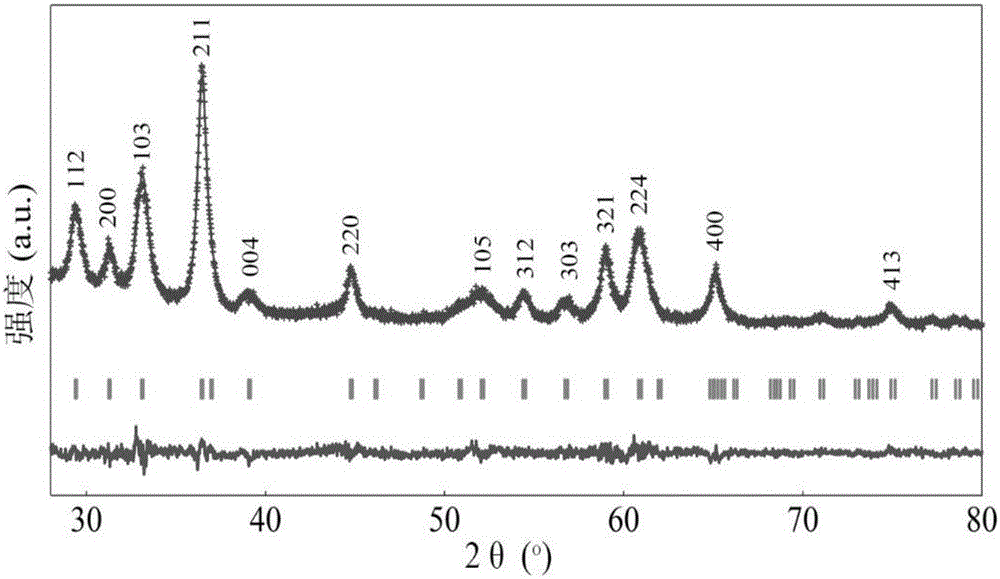
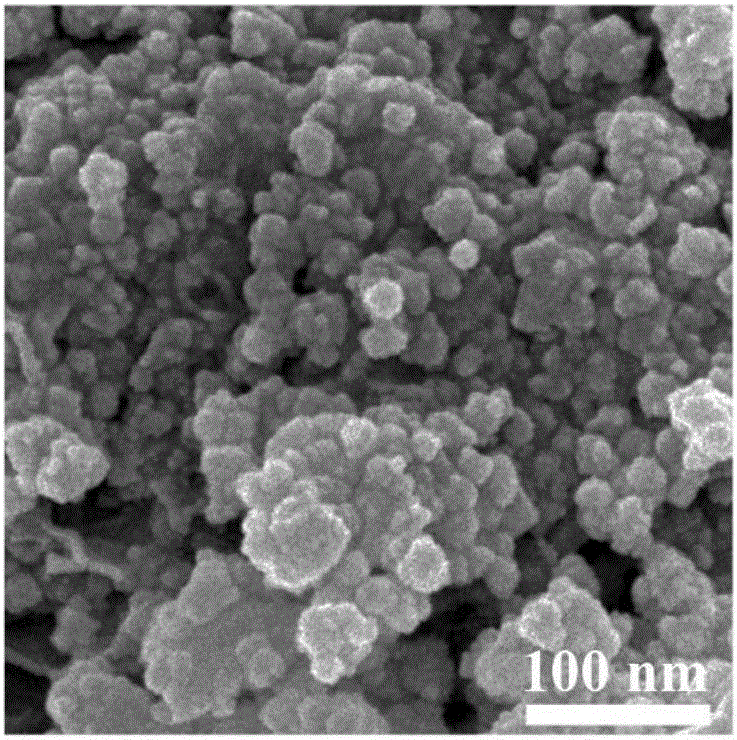
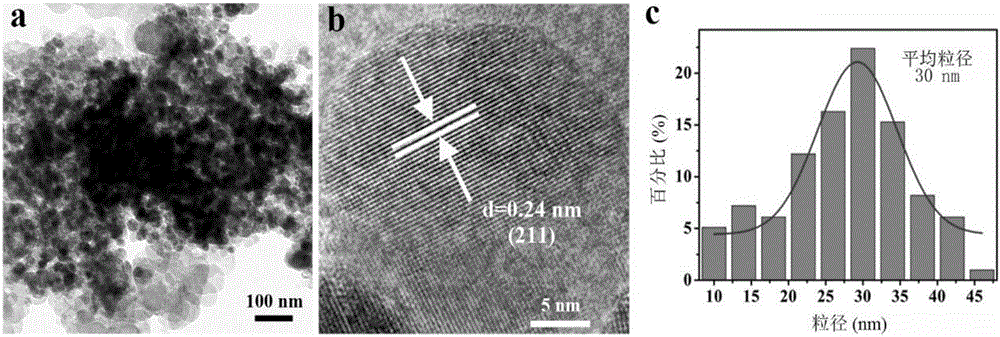
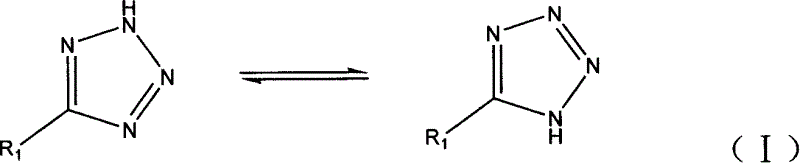
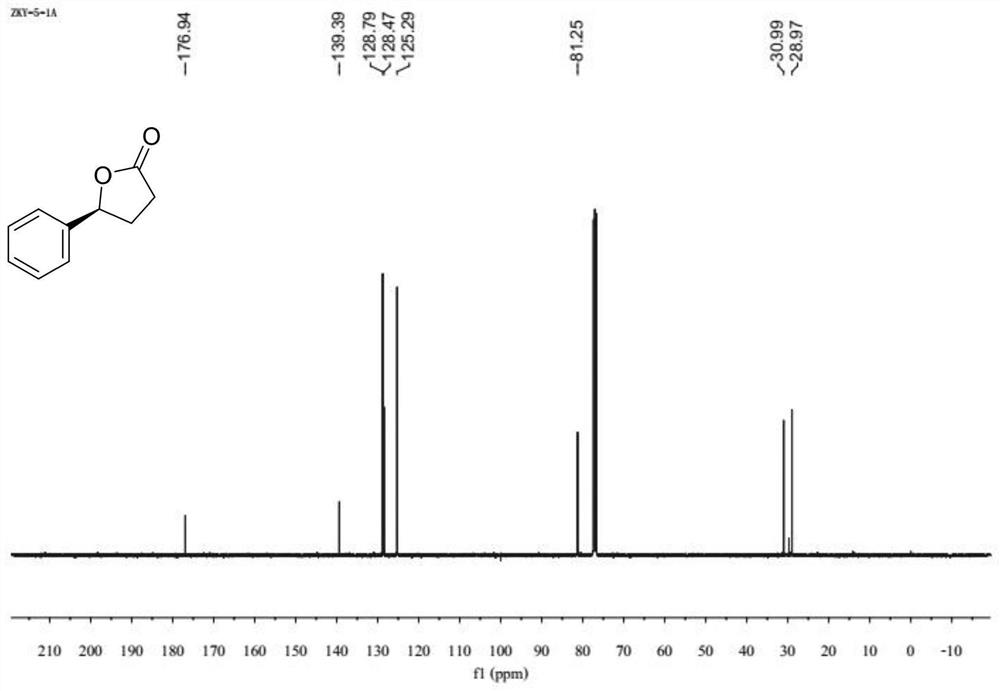
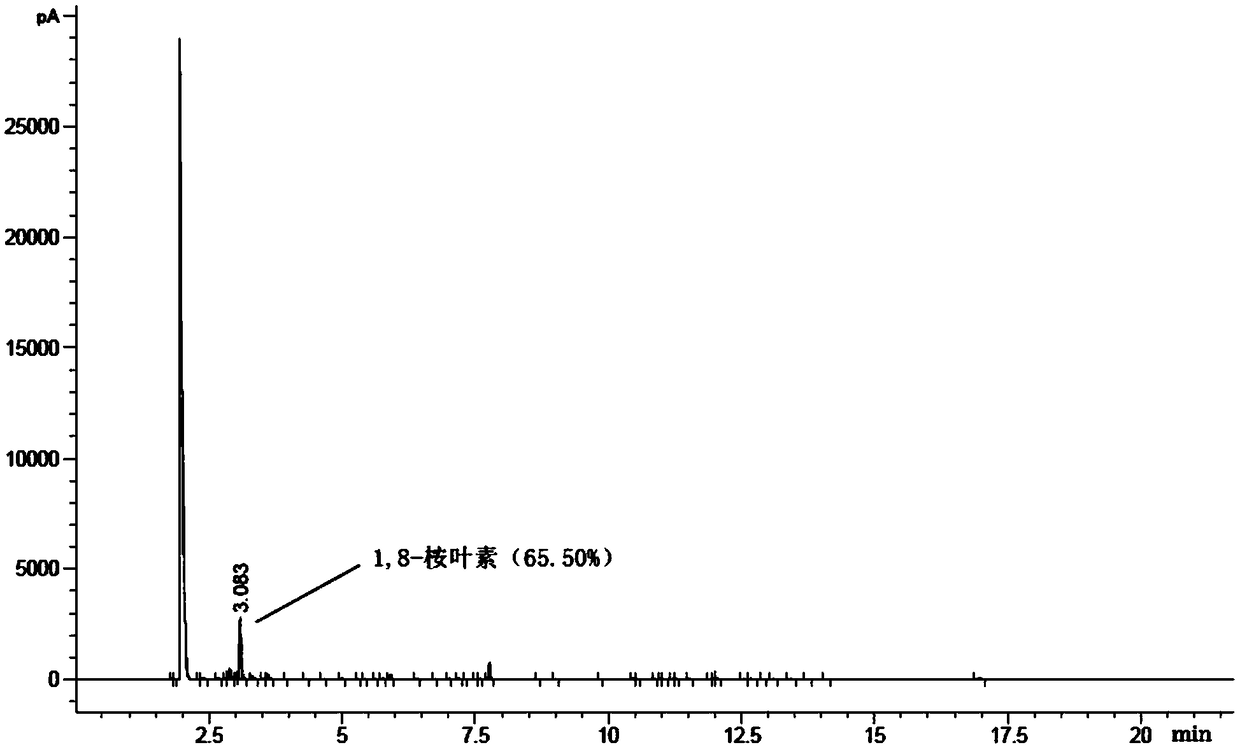
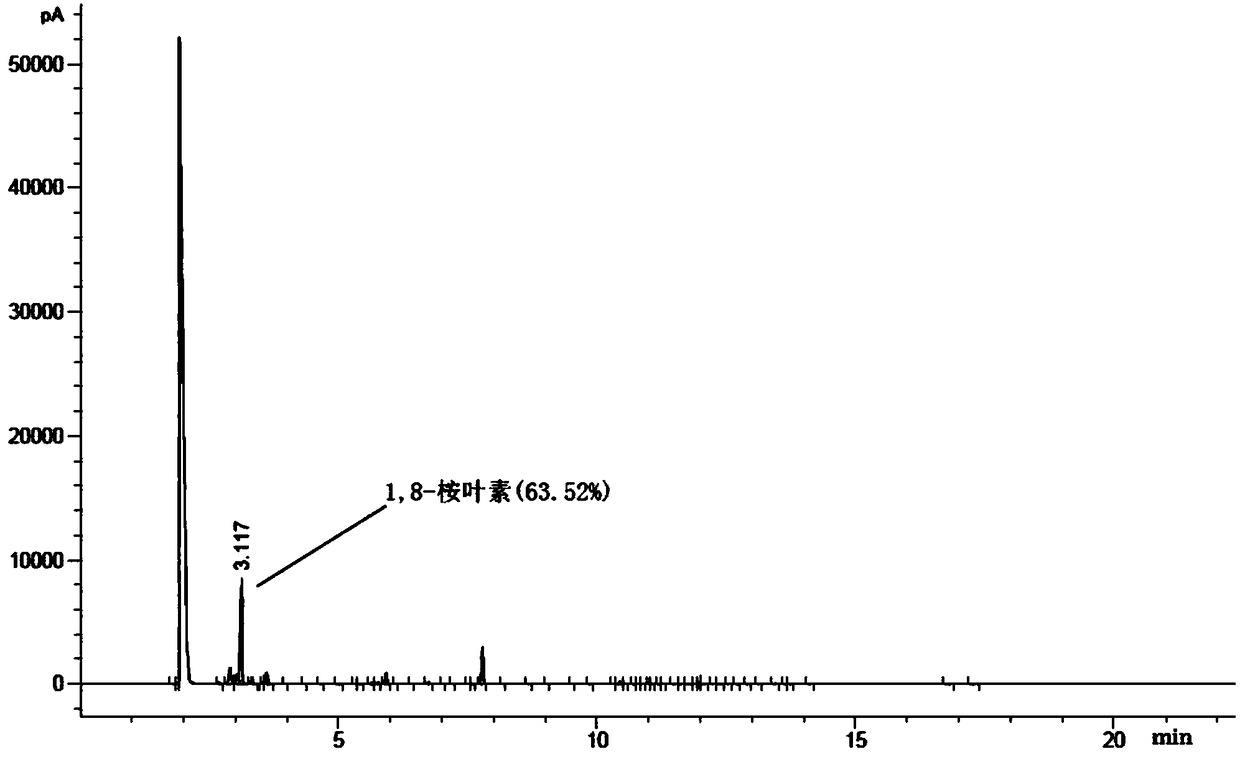
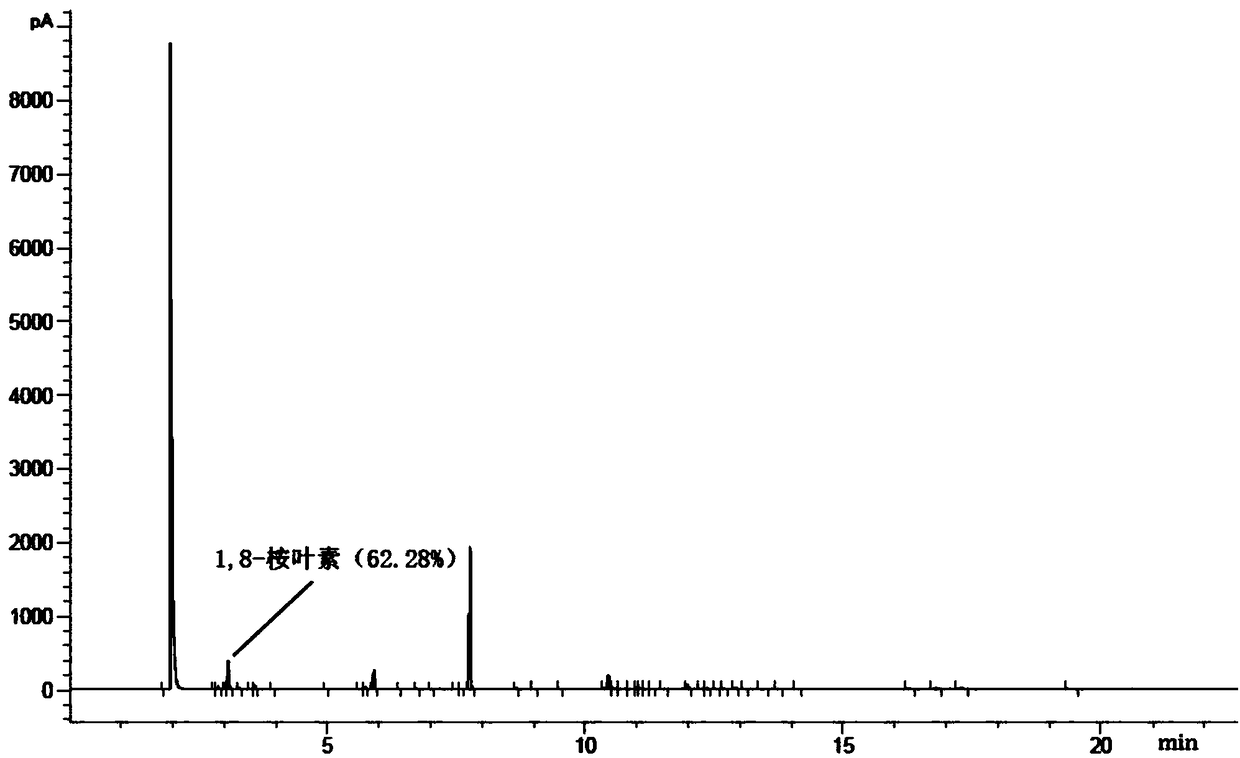
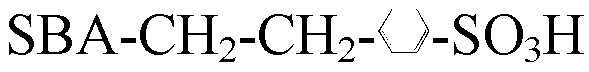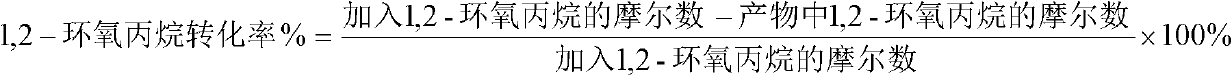Patents
Literature
42 results about "Zinc trifluoromethanesulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zinc trifluoromethanesulfonate or zinc triflate is the zinc salt of trifluoromethanesulfonic acid. It is commonly used as a Lewis acid catalyst, e.g. in silylations. A white powder, zinc triflate is commercially available, though some workers have experienced inconsistent results with zinc triflate from different sources.
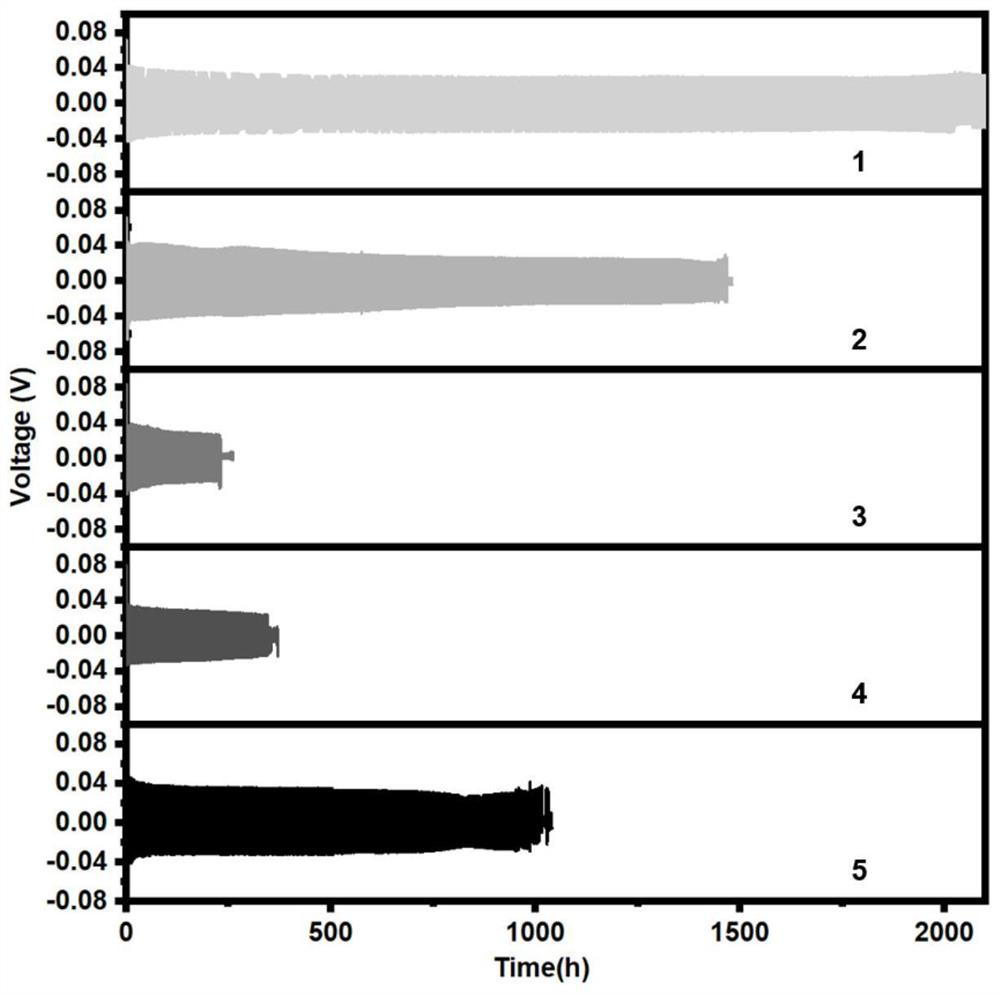
Chargeable water-system zinc ion battery with long cycle life and high energy density
ActiveCN105958131AIncrease energy densitySolution to short lifeFinal product manufactureCell electrodesCarbon compositesHigh energy
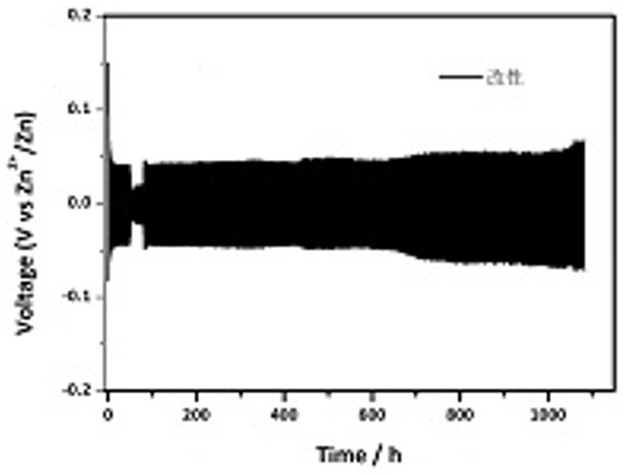
Disclosed is a chargeable water-system zinc ion battery with long cycle life and high energy density. The chargeable water-system zinc ion battery comprises a positive electrode shell, an elastic sheet, a gasket, a positive electrode active substance, a diaphragm, a negative electrode active substance and a negative electrode shell, and all the components form a laminated layer structure in sequence, wherein the positive electrode active substance is positive ion defect type ZnMn<x>O<4> / C nanocomposite; the negative electrode is zinc foil or spherical zinc powder; the diaphragm is polyethylene nonwoven fabric or filter paper; and the electrolyte is a zinc trifluoromethanesulfonate water solution. The chargeable water-system zinc ion battery has the advantages that the ZnMn<x>O<4> / conductive carbon composite electrode material has simple and easily feasible preparation process; the synthesized ZnMn<x>O<4> nano crystals are uniformly embedded in the conductive carbon; due to the electrolyte, the Zn deposition / separation-out coulombic efficiency can reach about 100% and a wide electrochemical window of 0-2.5Vvs.Zn<2+> / Zn is realized; the positive electrode active substance and the novel electrolyte are applied to the water-system zinc ion battery, so that the battery shows a good electrochemical performance; and meanwhile, the battery has high reversible zinc storage capacity with high active substance content, and excellent cycling stability .
Owner:NANKAI UNIV
Chemical synthesis method of 1,2,3,4-tetra nitroazole kind compound
InactiveCN1718574AHigh reaction yieldReduce manufacturing costOrganic chemistryChemical synthesisCompound a
Owner:ZHEJIANG UNIV OF TECH
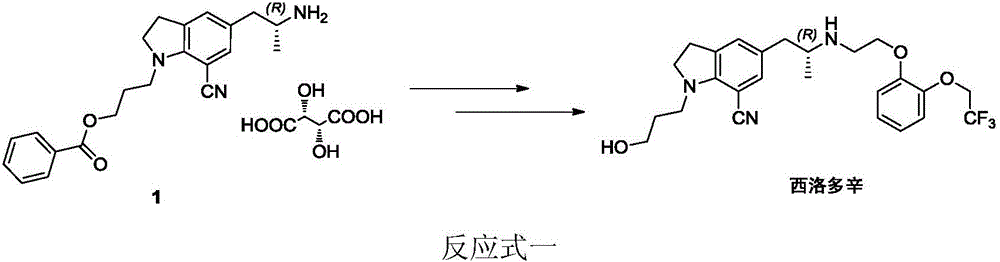
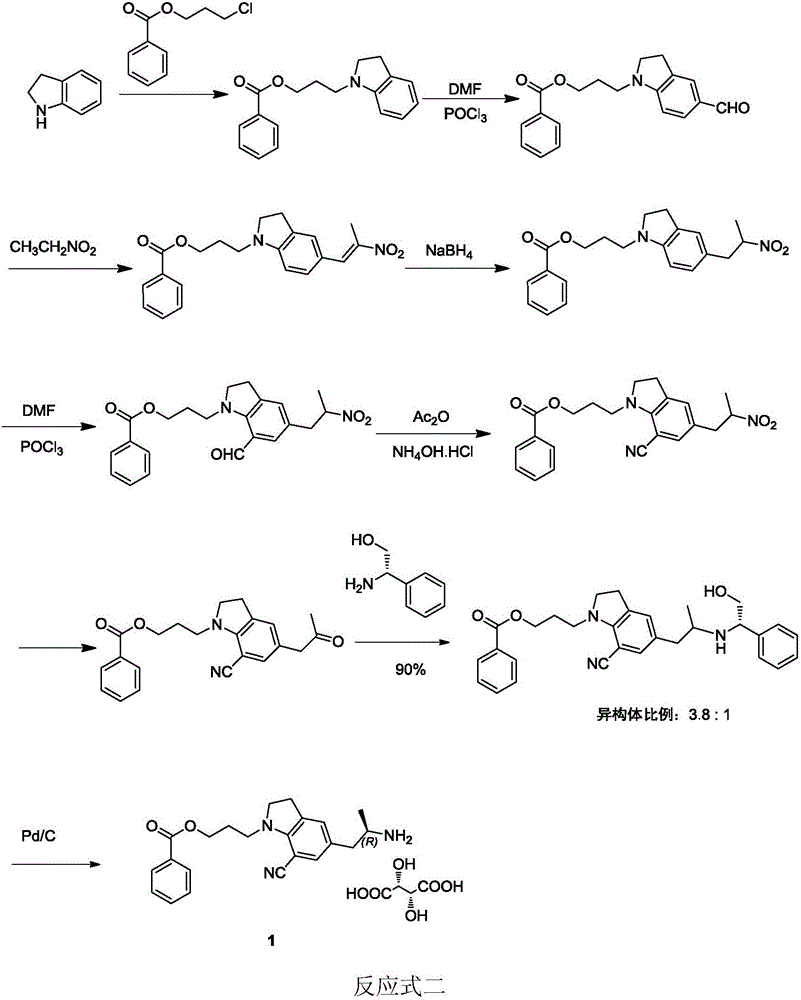
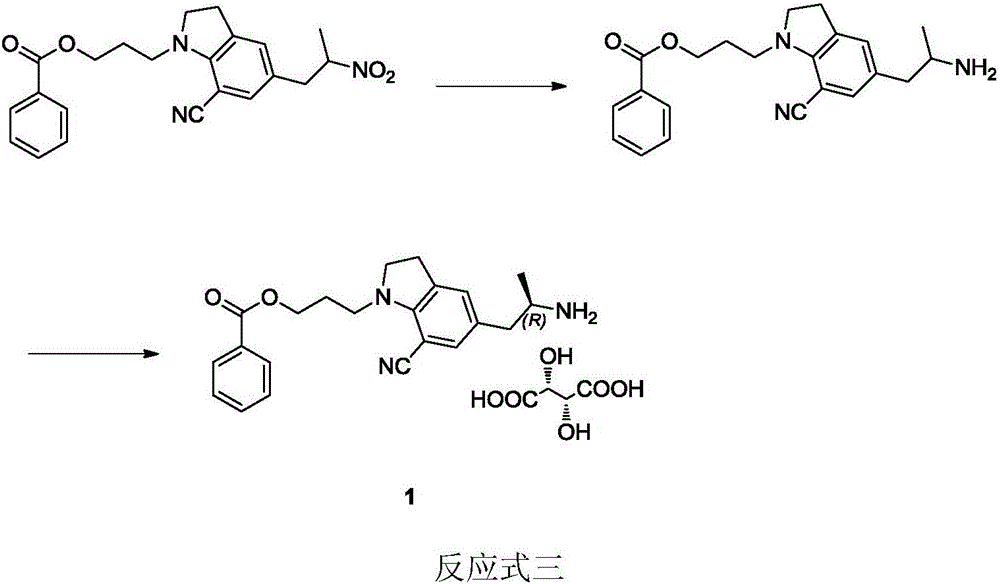
Preparation method of silodosin intermediate
The invention discloses a preparation method of a silodosin intermediate. The preparation method comprises the following steps that in an organic solvent, under the action of lewis acid, friedel-crafts acylation reaction occurs between a compound 2 and a compound 3, so as to obtain a compound 4, wherein the lewis acid is one of or more of zinc trifluoromethanesulfonate, bismuth trifluoromethanesulfonate, scandium trifluoromethanesulfonate and aluminum trichloride; under the action of organic acid or boron trifluoride ether complex, the compound 4 reacts with triethyl silicane, so as to obtain a compound 5; the compound 5 reacts with sodium azide, so as to obtain a compound 6; under the action of catalysts, the compound 6 reacts with di-tert-butyl dicarbonate ester and hydrogen, so as to obtain a compound 7; under an acidic condition, the deamination protective reaction of the compound 7 occurs, so as to obtain a compound 8; the compound 8 reacts with L-tartaric acid, so as to obtain a compound 1, namely the silodosin intermediate. The preparation method of the silodosin intermediate has the advantages of simplicity, economy and mild reaction conditions, and chiral resolution is not needed.
Owner:ZHEJIANG TIANYU PHARMA
Method for preparing a 1,3-dioxolane compound
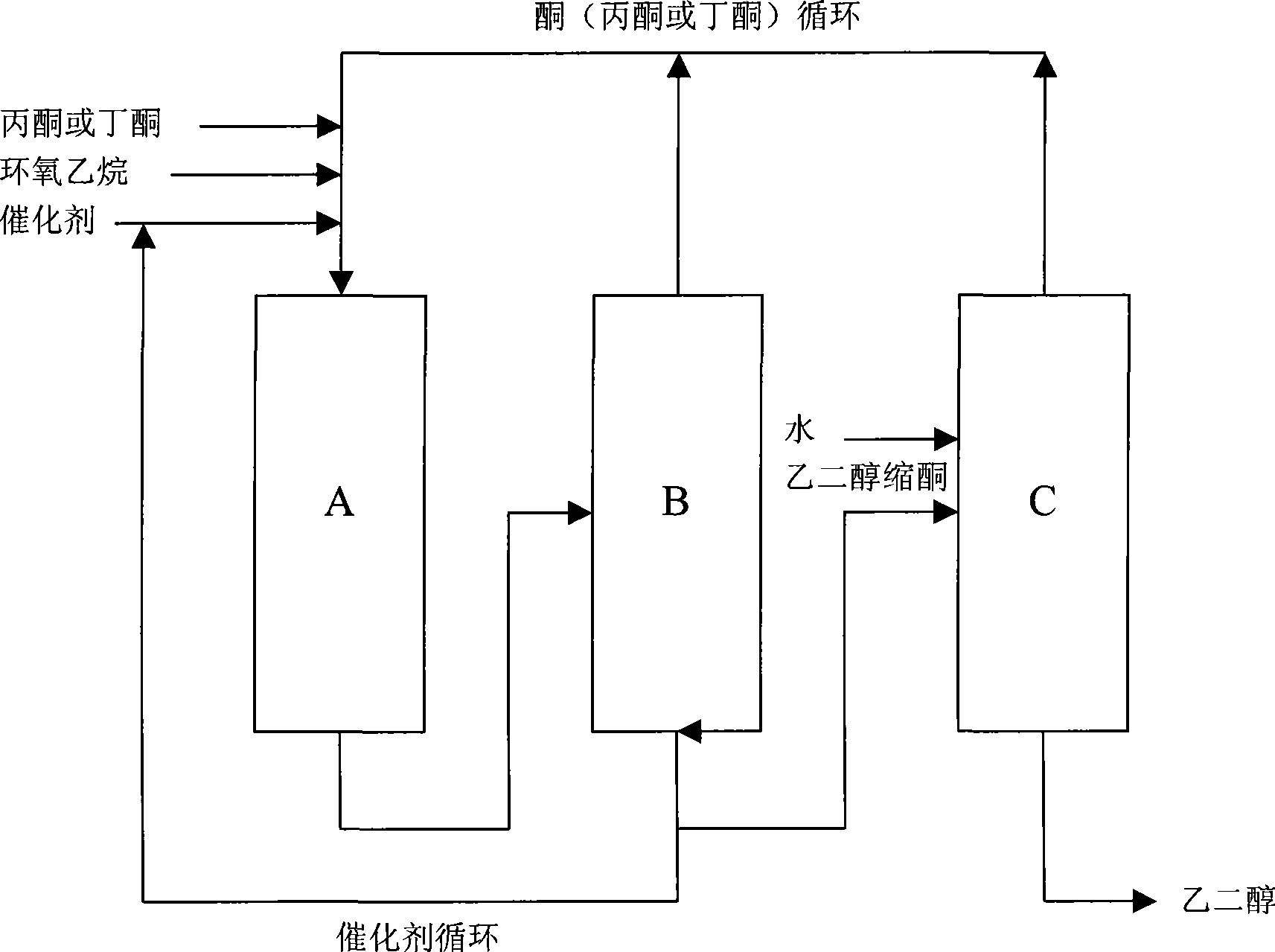
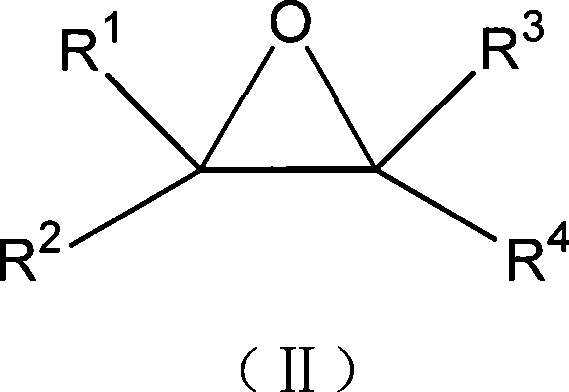
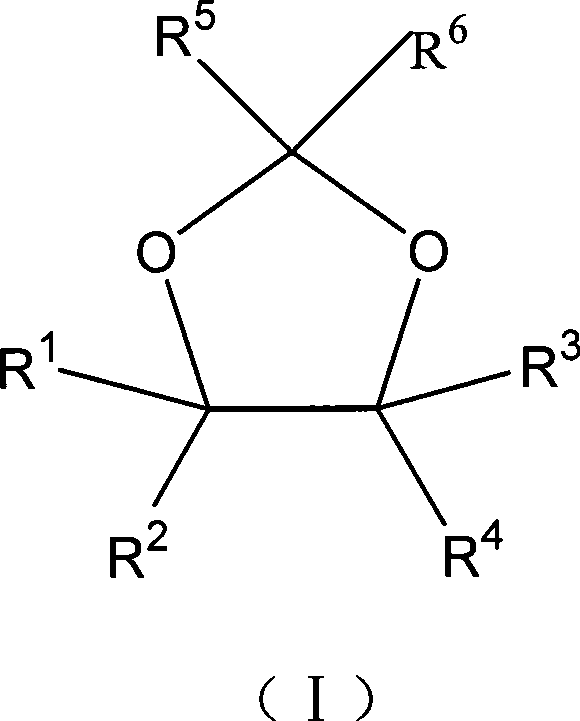
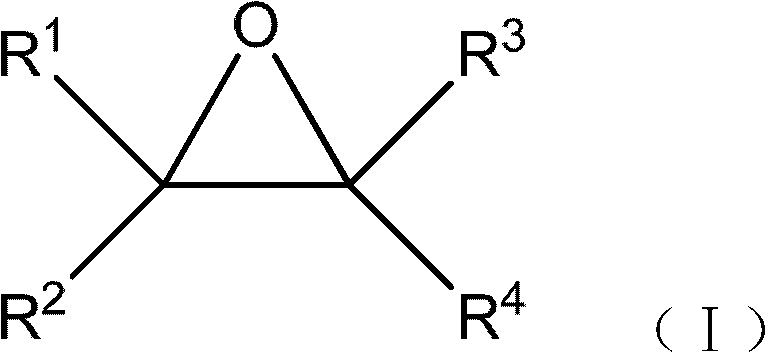
The invention relates to a method for preparing a 1, 3-dioxolane compound. With the catalysis of zinc trifluoromethanesulfonate, an epoxy compound reacts with ketone or aldehyde to generate the 1, 3-dioxolane compound and the rate of conversion is increased. The method can be applied to the development of a new production technology that ethylene alcohol is prepared by the hydrolysis of epoxy ethane in the field of petrochemical industry, save energy and reduce investment in equipment.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method of preparing polyaspartic ester
ActiveCN110981741AImprove conversion rateElectropositivity increaseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystIndium triflate
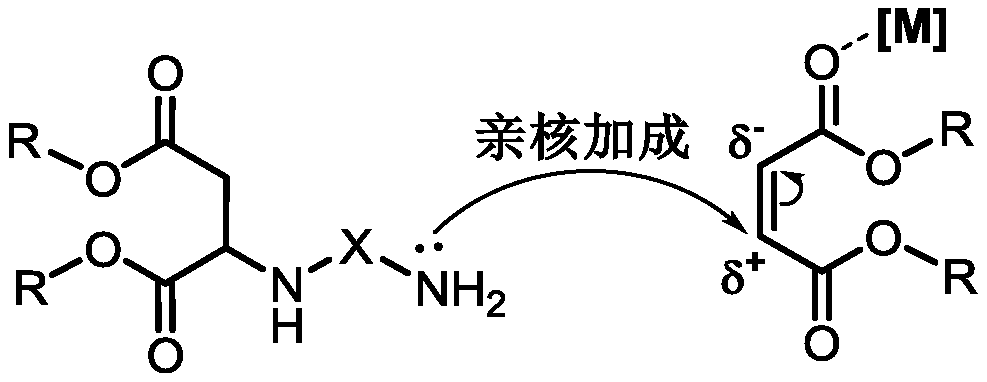
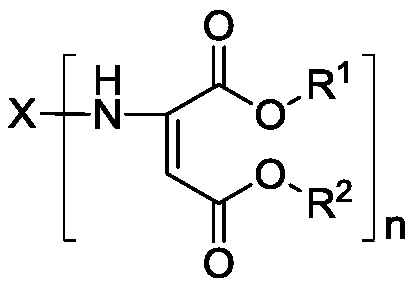
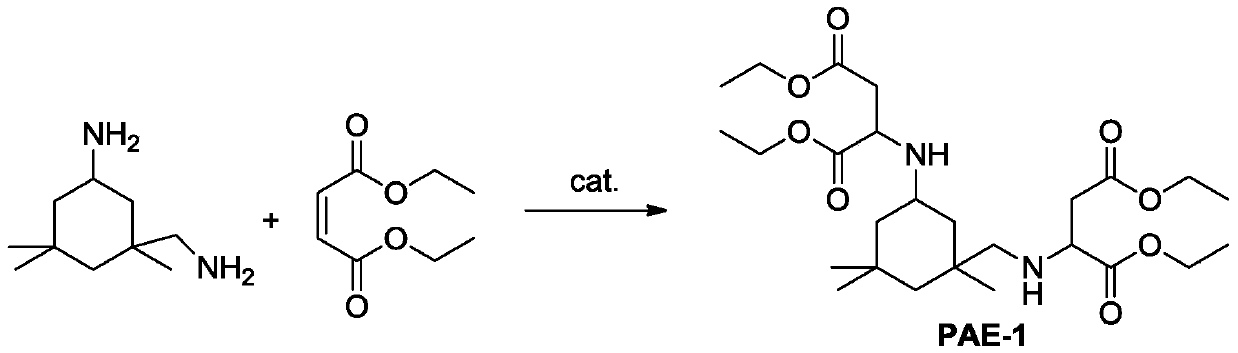
The invention discloses a method of preparing polyaspartic ester. The preparation method comprises the following steps: (1) organic primary amine and unsaturated dibasic acid ester are subjected to Michael addition reaction under the catalysis of a supported Lewis acid catalyst, and (2) a catalyst and excessive unsaturated dibasic acid ester are removed from the reaction solution obtained in the step (1) to obtain polyaspartic ester, wherein the supported Lewis acid catalyst comprises 40-60 wt% of indium trifluoromethanesulfonate, 10-30 wt% of one or more of indium chloride, copper trifluoromethanesulfonate, aluminum trifluoromethanesulfonate and zinc trifluoromethanesulfonate, and the balance of a carrier, and the content is based on the total weight of the catalyst. Raw materials of thecatalyst are common and easy to obtain, the preparation method is simple and convenient, and the method has the advantages of good catalytic activity, high primary amine conversion rate, simplicity and convenience in operation, high product quality and the like when used for preparing polyaspartic ester.
Owner:WANHUA CHEM GRP CO LTD
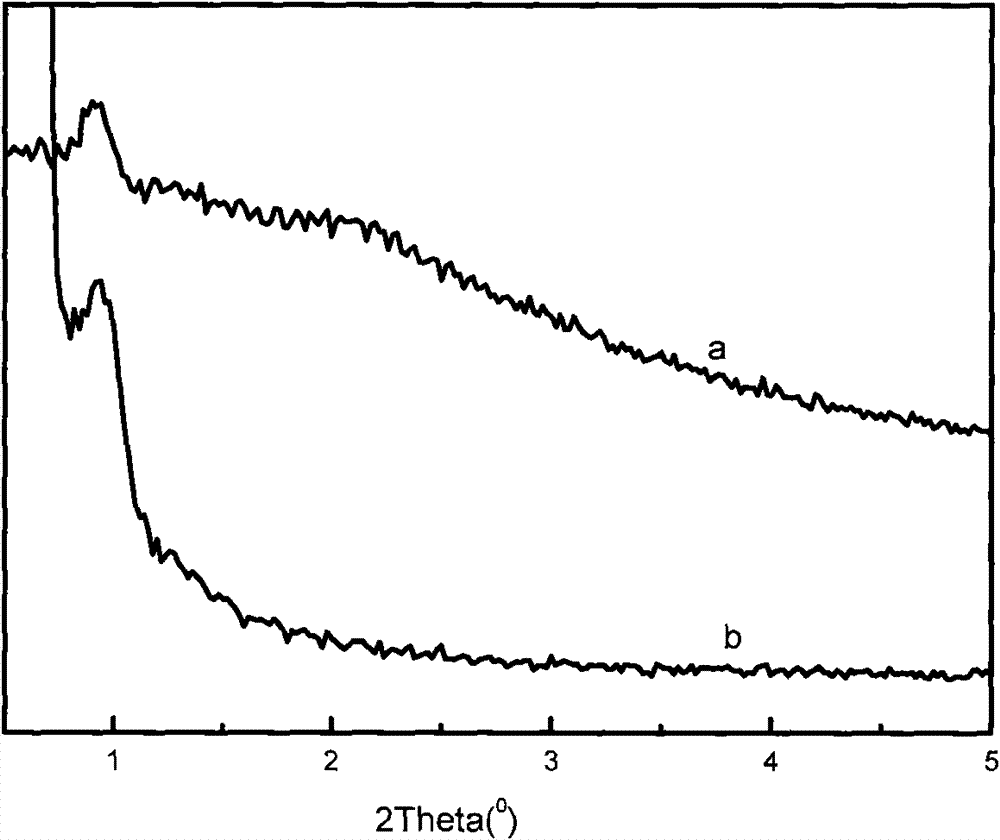
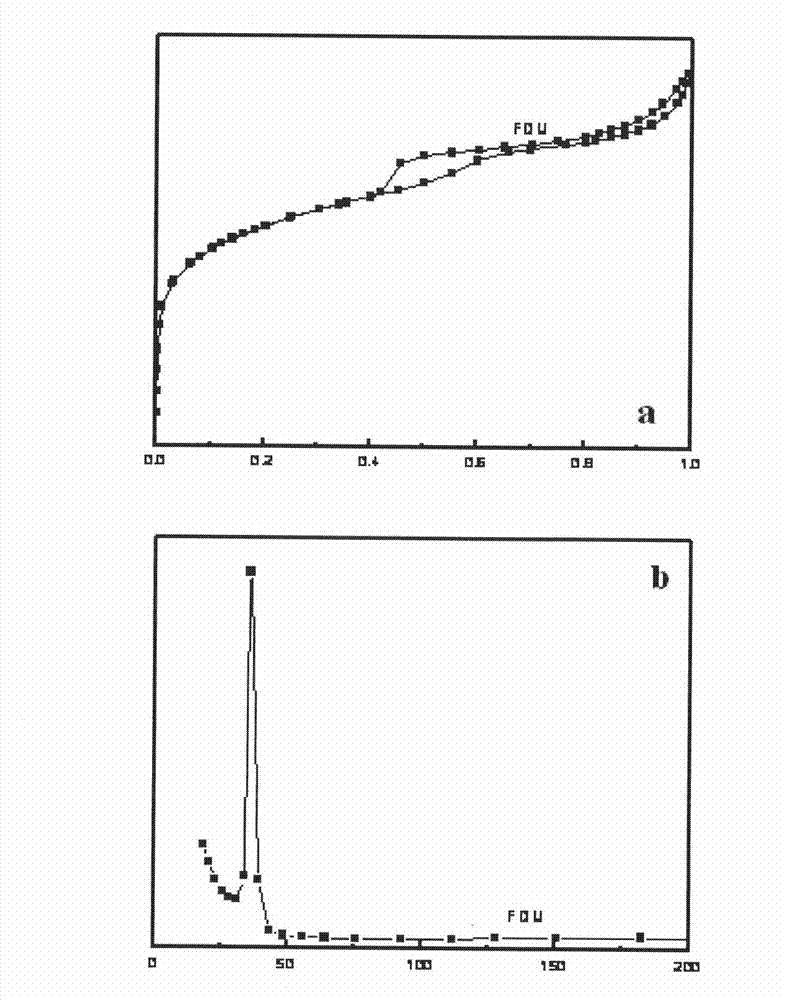
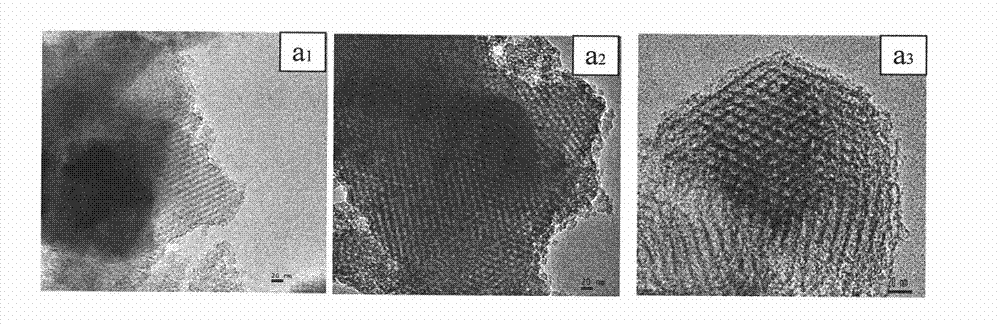
Zinc trifluoromethanesulfonate-loaded spherical mesoporous material, and preparation method and application thereof
ActiveCN102039177AImprove conversion rateReduce pollutionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCyclohexanonePropylene glycol preparation
The invention relates to the technical field of catalyst synthesis, in particular to a zinc trifluoromethanesulfonate-loaded spherical mesoporous material, and a preparation method and application thereof. The mesoporous material serves as a catalyst which is used for a reaction process for preparing cyclohexanone-1,2-propylene glycol from cyclohexanone and 1,2-propylene glycol. The mesoporous material has the advantages of high conversion rate of a reactant after a catalytic reaction, a small number of side reactions, high product purity, high conversion rate after repeated use and environmental friendliness.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for asymmetrically synthesizing dihydrofuran-2-(3H)-one compound under catalysis of nickel
The invention relates to a method for asymmetrically synthesizing a dihydrofuran-2-(3H)-one compound under the catalysis of nickel, wherein the method comprises the steps: by taking gamma-ketonic acid as a raw material, adding a nickel catalyst, a ligand and Lewis acid in an organic solvent environment in which hydrogen is taken as a hydrogen source at the pressure of 0.1-6 MPa and the temperature of 50-90 DEG C, and carrying out hydrogenation reaction to synthesize the dihydrofuran-2-(3H)-one compound. By adopting a transition metal-lewis acid synergistic catalysis strategy and taking nickel acetate tetrahydrate as a transition metal catalyst, the preparation method has the advantages of easiness in obtaining and low cost; zinc trifluoromethanesulfonate is added, so that the reaction temperature is greatly reduced from 150 DEG C to 70 DEG C, and compared with a metal catalytic reaction in the prior art, the reaction is milder. The method disclosed by the invention is wide (suitable) in application range, simple in catalytic system, simple and convenient to operate and high in yield and enantioselectivity (the yield is as high as 97%, and ee is as high as 95%), the production cost is greatly reduced, and the method has remarkable social benefits and economic benefits.
Owner:YUNNAN MINZU UNIV
Method for synthesizing 1,8-cineole from terpilenol
The invention discloses a method for synthesizing 1,8-cineole from terpilenol, and relates to the technical field of synthesis of 1,8-cineole. Terpilenol is converted into 1,8-cineole through heterogeneous catalysis with zinc trifluorosulfonate or copper trifluorosulfonate as the catalyst. Terpilenol, dichloromethane and a solid catalyst are added into a reaction bulb for a stirring reaction through a stirrer under the protection of nitrogen at normal temperature and normal pressure, and the interference of an external environment is avoided. Dichloromethane diluted reaction liquid is added after the reaction is completed, a saturated sodium bicarbonate solution is added for extraction, a dichloromethane phase is synthesized and washed three times with a saturated salt solution, anhydroussodium sulfate is added for drying, and an organic phase is concentrated to obtain 1,8-cineole with certain purity. It is verified through experiments that the terpilenol conversion rate is up to 98%and the effective conversion rate of 1,8-cineole reaches 60% or above. Terpilenol is low in price and easy to obtain when serving as the raw material, and the production cost is reduced; high temperature, high pressure and other strict conditions are avoided, the technological steps are simple, the reaction time is short, and the conversion rate is high; no harmful byproducts can be generated, andthe method is friendly to the environment.
Owner:YUNNAN LORRAINE AROMATIC PROD CO LTD
Preparation process of zinc trifluoromethanesulfonate
InactiveCN112239419AImprove production efficiencyHigh purityThiol preparationOrganic compound preparationTriflic acidSulfuryl
Owner:JIANGXI GUOHUA IND CO LTD
Aqueous zinc ion battery additive, electrolyte prepared from aqueous zinc ion battery additive and application of aqueous zinc ion battery additive
PendingCN114447446ALow priceHydrophilicFinal product manufactureSecondary cellsElectrolytic agentSulfate zinc
The invention relates to an aqueous zinc ion battery additive, an electrolyte containing the additive and application, the additive is one or more of methanol, ethanol and ethylene glycol, and the electrolyte comprises a solvent and a solute. Wherein the solvent is water, the solute is one or more of zinc trifluoromethanesulfonate, zinc sulfate and zinc chloride, and the volume ratio of the additive to the solvent water is 1: (1-10). The additive provided by the invention can be mixed with water in any proportion, and can form hydrogen bonds with water. The interaction between the additive provided by the invention and water can effectively inhibit the adverse reaction in the water-based zinc ion battery, thereby improving the electrochemical performance of the water-based zinc ion battery.
Owner:QILU UNIV OF TECH
Aqueous zinc ion battery electrolyte for inhibiting vanadium dissolution of vanadium-based positive electrode as well as preparation method and application thereof
InactiveCN113097577AReduce in quantityInhibitory activityFinal product manufactureSecondary cells servicing/maintenanceElectrolytic agentZinc ion
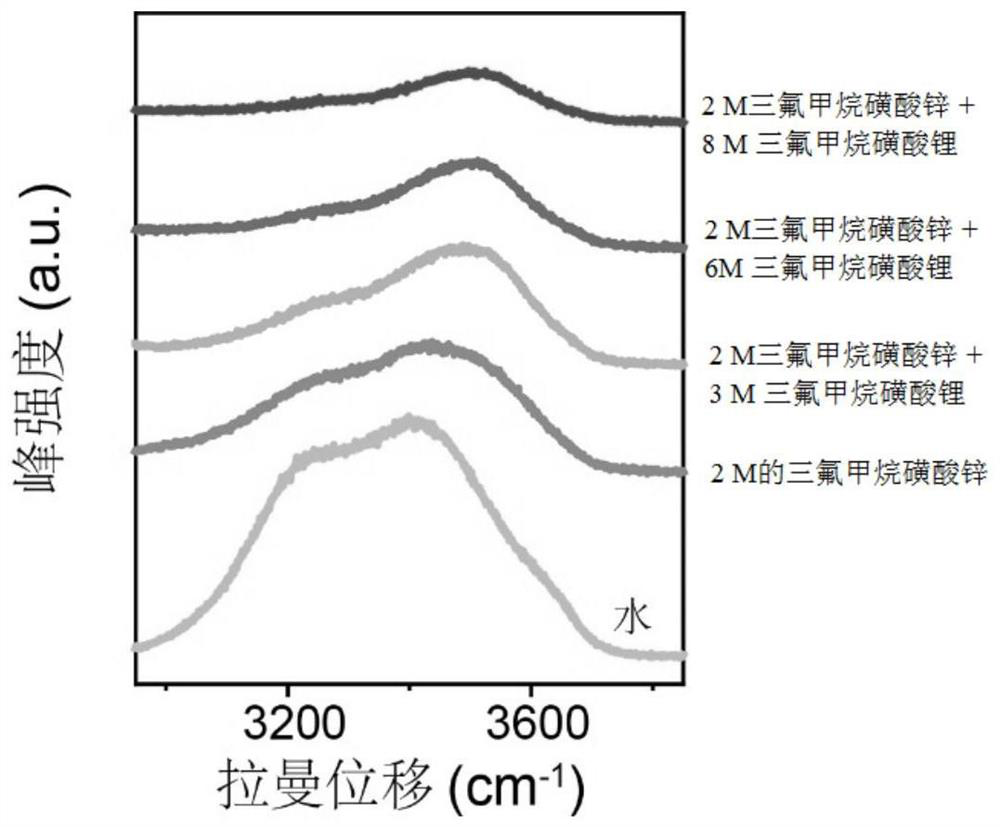
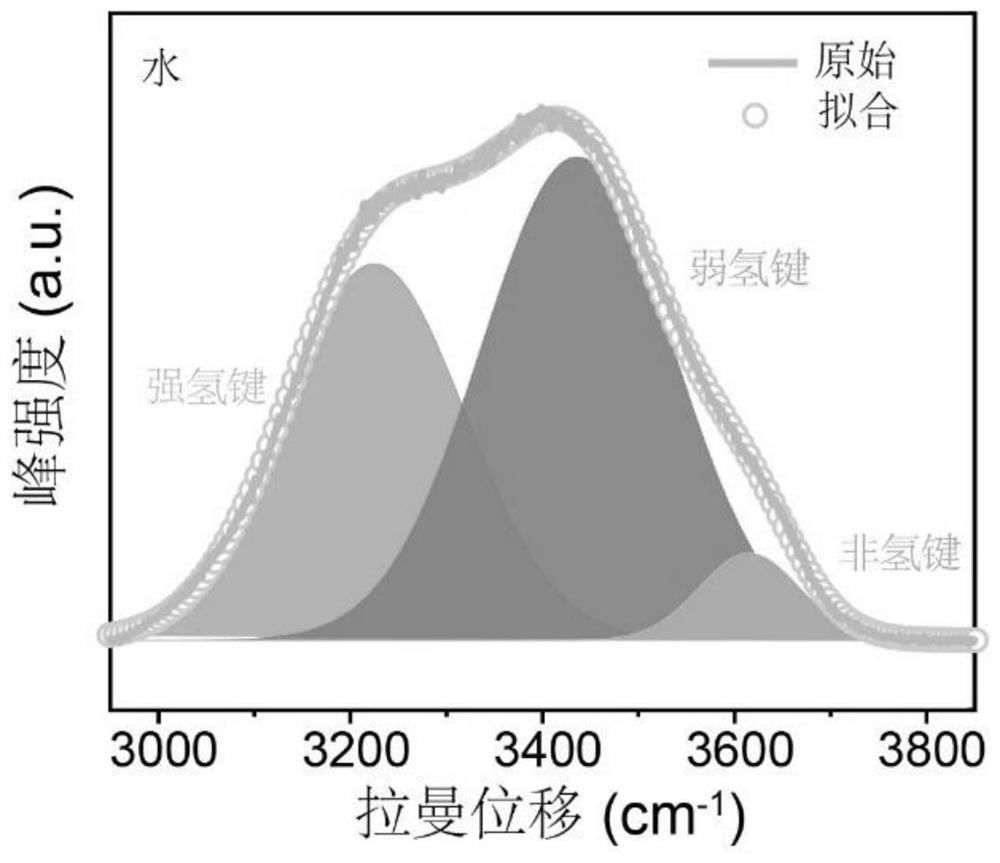
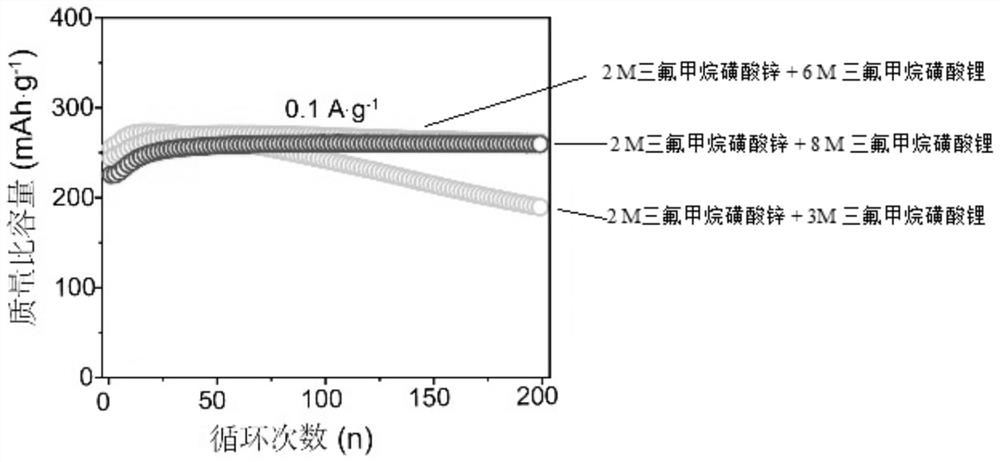
The invention provides an aqueous zinc ion battery electrolyte for inhibiting vanadium dissolution of a vanadium-based positive electrode as well as a preparation method and application of the aqueous zinc ion battery electrolyte. The aqueous zinc ion battery electrolyte comprises the following components: zinc trifluoromethanesulfonate (Zn (CF3SO3) 2), lithium trifluoromethanesulfonate (LiCF3SO3) and water. Through combination of the zinc trifluoromethanesulfonate and the lithium trifluoromethanesulfonate, on one hand, the number of free water molecules in the electrolyte can be effectively reduced, the activity of the water molecules is inhibited, and the stability of an electrode interface is improved; and on the other hand, the solvation structure of hydrated zinc ions in the aqueous electrolyte can be changed, the number of coordinated water molecules is reduced, the number of intercalated water molecules is further reduced, and the lattice main structure is effectively stabilized. The electrolyte is used for being matched with the vanadium-based positive electrode of the aqueous zinc ion battery, so that the cycling stability of the positive electrode under small current is remarkably improved, and the purpose of inhibiting vanadium dissolution is achieved.
Owner:GUANGDONG UNIV OF TECH
Preparation method and application of metal organic cage compound
ActiveCN111454249AHigh yieldChemically stableOrganic chemistryOrganic compound preparationTetrafluoroborateBoronic acid
The invention belongs to the technical field of fine chemical engineering, and relates to a preparation method and application of a metal organic cage compound. According to the preparation method, Zn<2+> in transition metal salt is used as a node, L is used as a ligand, and a reaction is conducted to prepare the metal organic cage compound; a synthetic route is that Zn<2+> and L produce Zn-L; theligand L is H<2>ZPA; and the transition metal salt is one selected from a group consisting of zinc perchlorate, zinc nitrate, zinc tetrafluoroborate and zinc trifluoromethanesulfonate. The metal organic cage compound prepared by using the method is low in raw material price, high in yield, stable in chemical property, easy to put into practical application, and capable of efficiently catalyzing cyclobutanone to prepare butyrolactone and catalyzing selective oxidation of thioether to prepare sulfoxide under the condition of mild illumination.
Owner:DALIAN UNIV OF TECH
Aqueous zinc ion battery electrolyte as well as preparation method and application thereof
PendingCN113314773AImprove electrochemical cycle stabilityUniform depositionFinal product manufactureSecondary cells servicing/maintenanceSolid state electrolyteElectrolytic agent
The invention relates to an aqueous zinc ion battery electrolyte. The electrolyte is composed of a matrix electrolyte and a high-adhesion organic matter containing a catechol functional group, the high-adhesion organic matter containing the catechol functional group is one of catechol, [alpha]-methyldopa, [alpha]-methyldopamine, noradrenaline, gallic acid, gallocatechin, 6-hydroxydopamine, dihydroxyphenylacetamide, dihydroxyphenylethyl thiourea, dihydroxyindoline, dihydroxytryptamine, trihydroxyindole, catechin and tannic acid. The preparation method comprises the following steps: dissolving one of zinc sulfate, zinc chloride and zinc trifluoromethanesulfonate in deionized water, and stirring and dissolving at normal temperature to prepare a matrix electrolyte; d anadding the organic matter into the matrix electrolyte to prepare the electrolyte. The electrolyte can form a stable mussel bionic solid electrolyte interface film on the surface of a zinc negative electrode in situ, dendrite-free deposition of zinc is induced, the cycle performance and coulombic efficiency of the zinc negative electrode are improved, and the electrochemical performance of a zinc ion battery is improved.
Owner:XUZHOU NORMAL UNIVERSITY
Preparation method of organic porous material
ActiveCN109876778AImprove thermal stabilityGood chemical stabilityOther chemical processesDispersed particle separationIndiumThiourea
The invention provides a preparation method of an organic porous material. The preparation method includes the following steps that ion-like liquid and 2,4,6-trihydroxy-1,3,5-benzene trioxin are addedinto a reaction vessel, then a catalyst is added to be subjected to ultrasonic processing and mixing evenly, the reaction vessel is placed in liquid nitrogen to be frozen for 1-3 min, vacuumized to 0.01-1 pa, and sealed, and a product is obtained through a reaction for 24-72 h at 90-150 DEG C; the ion-like liquid is prepared from a hydrogen bond donor and a hydrogen bond receptor, and the hydrogen bond donor is one of carbamide and thiourea; the catalyst is one of scandium trifluoromethanesulfonate, europium trifluoromethanesulfonate, indium trifluoromethanesulfonate, ytterbium trifluoromethanesulfonate hydrate, yttrium trifluoromethanesulfonate, and zinc trifluoromethanesulfonate; the product is washed through a solvent, and filtered after Soxhlet extraction for 12-48 h; and the productis dried under the vacuum condition to obtain the organic porous material. According to the preparation method of the organic porous material, use of volatile and toxic organic solvents can be avoided, and environmental-friendly performance is improved.
Owner:CHINA UNIV OF MINING & TECH
Aqueous zinc ion battery electrolyte and battery
PendingCN114388901ALow costSimple preparation processSecondary cellsAqueous electrolytesElectrolytic agentTetrafluoroborate
The invention provides an aqueous zinc ion battery electrolyte and a battery, and belongs to the field of aqueous zinc ion batteries, the aqueous zinc ion battery electrolyte comprises sodium glutamate, zinc salt and water, the concentration of the sodium glutamate in the aqueous zinc ion battery electrolyte is 0.01 mol / L-0. 5mol / L, preferably 0.1 mol / L-0. 5mol / L, and the concentration of the zinc salt in the aqueous zinc ion battery electrolyte is 0.01 mol / L-0. 5mol / L, preferably 0.1 mol / L-0. 5mol / L, preferably 0.1 mol / L-0. 5mol / L; the zinc salt is selected from one or more of zinc sulfate, zinc chloride, zinc nitrate, zinc acetate, zinc perchlorate, zinc tetrafluoroborate and zinc trifluoromethanesulfonate, and the concentration of the zinc salt in the aqueous zinc ion battery electrolyte is 1.5 mol / L to 2.5 mol / L. The invention also provides an aqueous zinc ion battery which comprises a positive electrode, a negative electrode and an electrolyte, and the electrolyte is the aqueous zinc ion battery electrolyte. The problems of corrosion and zinc dendritic crystals of the zinc negative electrode are effectively solved, the cycling stability can be remarkably improved, and the cycling life can be remarkably prolonged.
Owner:HUAZHONG UNIV OF SCI & TECH
A kind of composite catalyst for propiconazole cyclization reaction and preparation method thereof
ActiveCN109833916BImprove the reaction medium environmentHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPropiconazole
The invention relates to a composite catalyst for propiconazole cyclization reaction and a preparation method thereof. The raw material composition and parts by weight of the composite catalyst for propiconazole cyclization reaction are: 1.5 parts of zinc trifluoromethanesulfonate to 3.5 parts, 0.8 parts to 2.2 parts of sodium trinitrobenzenesulfonate, 0.6 parts to 1.4 parts of sodium dichromate, 9.5 parts to 10.6 parts of catalyst carrier; Sodium benzene sulfonate and sodium dichromate are used together to make a composite catalyst through the catalyst carrier, which has good stability and excellent performance, so it can improve the reaction medium environment of the propiconazole cyclization reaction, improve the propiconazole cyclization reaction rate and ring The yield of the compound, the recovery is convenient and thorough, does not bring impurities into the subsequent bromination reaction, does not affect the yield of the bromination reaction, and improves the yield and purity of the final propiconazole technical drug.
Owner:JIANGSU HEBEN BIOCHEM +1
Method for preparing zwitter-ion hydrogel electrolyte
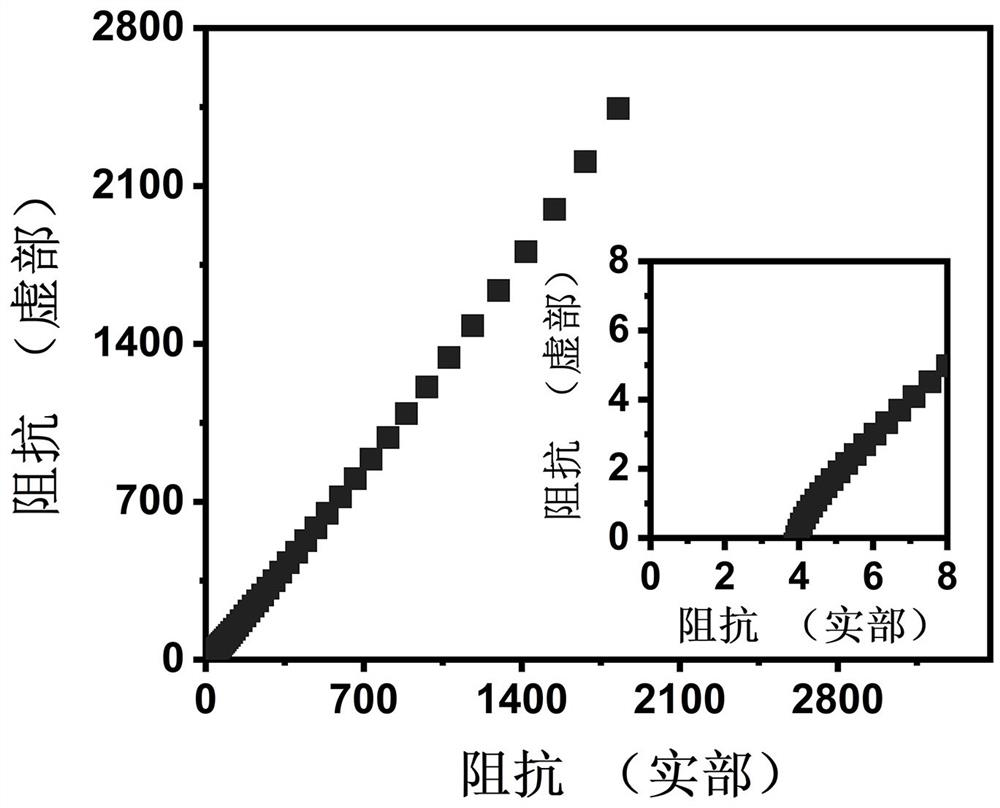
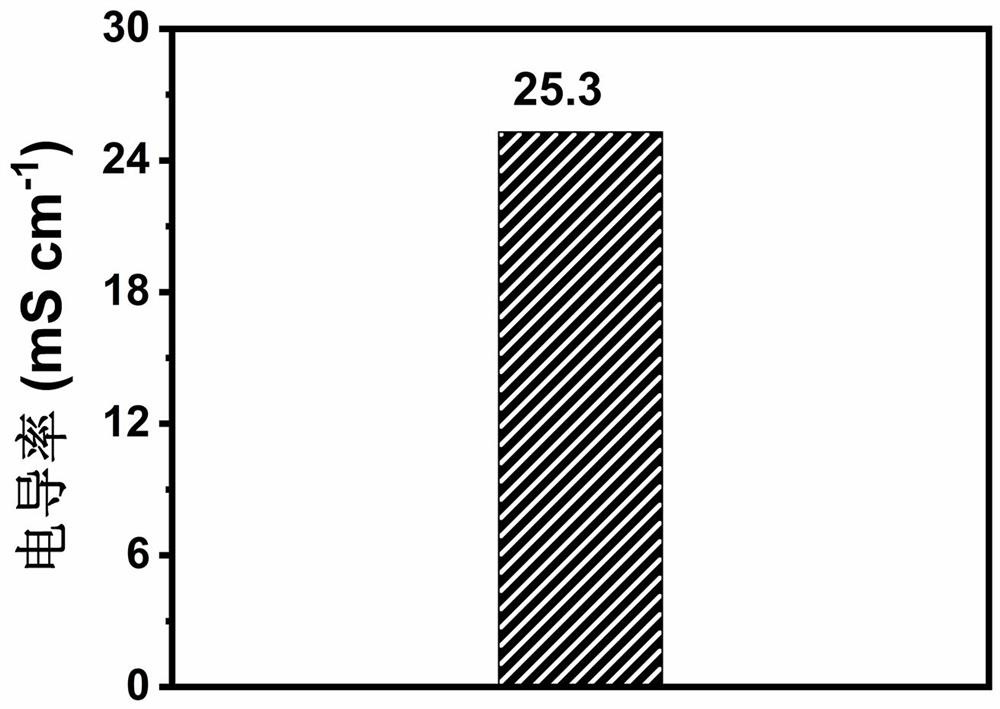
The invention discloses a synthetic method of CMC-P (MAEDS-co-AA) polymer zwitter-ion hydrogel and a preparation method of the polymer zwitter-ion hydrogel. The preparation method comprises the following steps: taking carboxymethyl cellulose, acrylic acid and [2-(methylacryloyloxy) ethyl] dimethyl-(3-sulfopropyl) ammonium hydroxide as raw materials, dissolving, and carrying out thermal polymerization to synthesize the hydrogel electrolyte. After the hydrogel electrolyte is soaked in a zinc trifluoromethanesulfonate solution for one hour, the hydrogel electrolyte has the excellent ionic conductivity of 25.3 mS cm < 1 >. The obtained hydrogel electrolyte is assembled into a quasi-solid-state zinc ion hybrid capacitor for electrochemical performance testing, the current density is 0.25-20 A g <-1 > during testing, and the voltage range is 0.2-1.8 V. The preparation method is simple and convenient, the microstructure of the synthetic material is of a porous structure, and the assembled quasi-solid zinc ion hybrid capacitor shows excellent specific capacity and rate capability.
Owner:XINJIANG UNIVERSITY
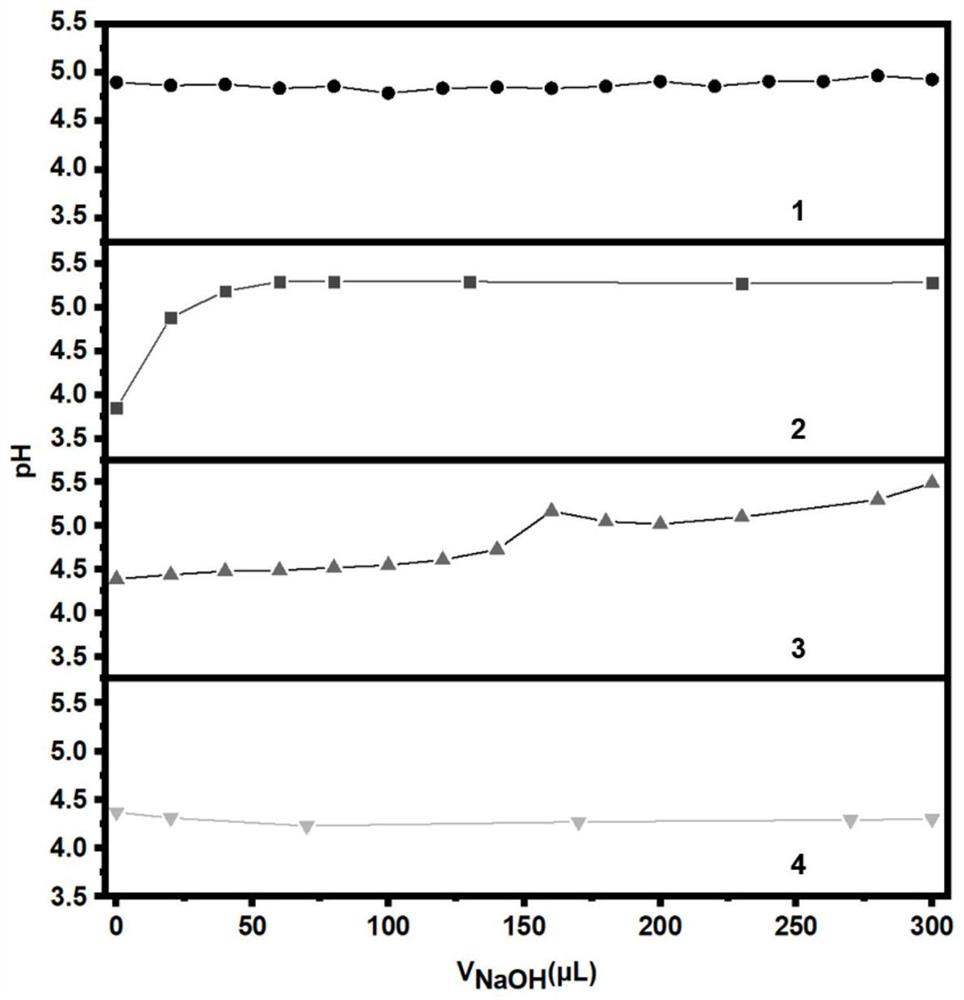
Mixed aqueous zinc ion battery electrolyte with stable pH value and application
PendingCN114865110AStable pHAvoid direct contactFinal product manufactureSecondary cells servicing/maintenanceElectrolytic agentSulfate zinc
The invention discloses a mixed aqueous zinc ion battery electrolyte with a stable pH value and application, and relates to an electrolyte and application. The invention aims to solve the problem that the pH stability, high coulombic efficiency and cycle life of an electrolyte cannot be realized at the same time in the existing aqueous zinc ion battery. The mixed aqueous zinc ion battery electrolyte with the stable pH value is formed by mixing zinc sulfate, zinc trifluoromethanesulfonate and ultrapure water; the method is applied to the water-based zinc ion battery. The invention provides the mixed aqueous zinc ion battery electrolyte with the stable pH value and the application of the mixed aqueous zinc ion battery electrolyte.
Owner:JILIN UNIV
Composite catalyst for propiconazole cyclization reaction and preparation method thereof
ActiveCN109833916AImprove the reaction medium environmentHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPropiconazoleTriflic acid
A composite catalyst for propiconazole cyclization reaction and a preparation method thereof are disclosed, the composite catalyst is prepared from the following raw materials in parts by weight: 1.5-3.5 parts of zinc trifluoromethanesulfonate, 0.8 to 2.2 parts of sodium trinitrobenzene sulfonate, 0.6 to 1.4 parts of sodium dichromate and 9.5-10.6 parts of a catalyst carrier; according to the preparation method disclosed by the invention, the zinc trifluoromethanesulfonate, the sodium trinitrobenzene sulfonate and the sodium dichromate are compounded together through the catalyst carrier to prepare the composite catalyst, and the composite catalyst is good in stability and excellent in performance, so that the reaction medium environment of the propiconazole cyclization reaction can be improved, the rate of the propiconazole cyclization reaction and the yield of a cyclization substance are increased, the recovery is convenient and thorough, no impurities can be brought to the subsequent bromination reaction, the yield of the bromination reaction is not affected, and the yield and purity of the final propiconazole original medicine are improved.
Owner:JIANGSU HEBEN BIOCHEM +1
Loaded zinc trifluoromethanesulfonate catalyst, its preparation method, and preparation method of butanone-glycol ketal
ActiveCN102451756BImprove catalytic performanceLess side effectsOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingButanoneMesoporous silica
Owner:CHINA PETROLEUM & CHEM CORP +1
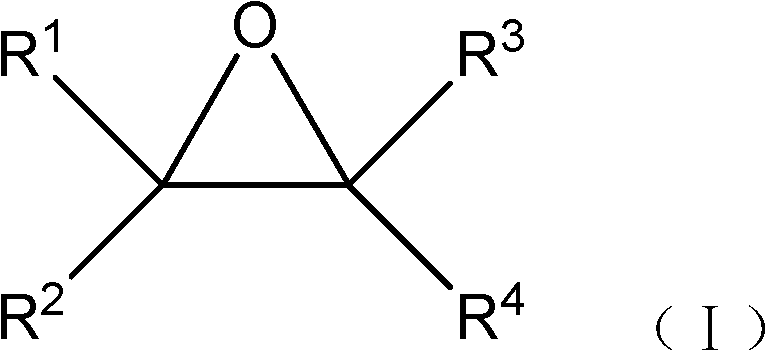
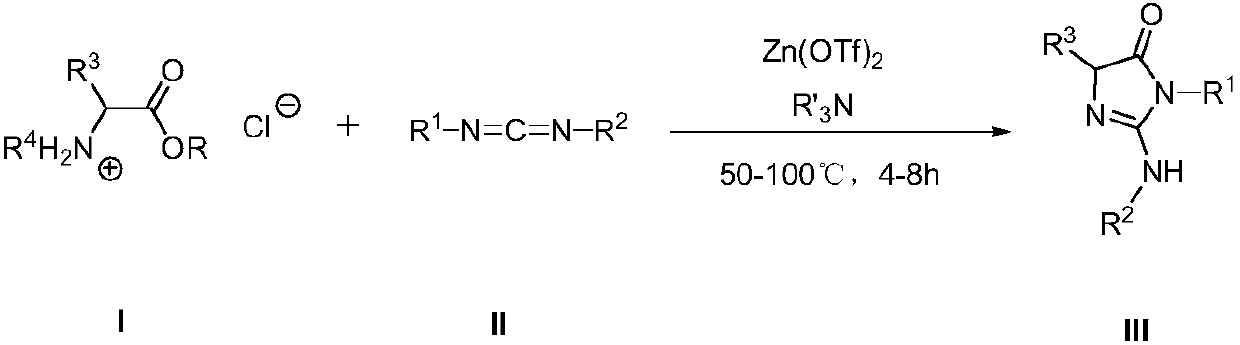
Synthesis method and application of imidazole derivative
ActiveCN105859630AWide range of usesHigh yieldOrganic chemistrySynthesis methodsZinc trifluoromethanesulfonate
The invention provides synthesis method and application of an imidazole derivative. The method uses carbodiimide, hydrochloride salt of amino-acid ester, tertiary amine and zinc triflate in a catalytic amount to prepare 2-amino or imino substituted imidazole derivative. The synthesis method is scientific and rational, provides a general method for synthesis of an imidazole derivative with a variety of substituents, and can further synthesize more complex heterocyclic molecules, such as benzimidazole imidazolone. The method has the advantages of easily available raw materials, wide range of application, high separation yield, simple laboratory equipment and operations, is convenient for further development and application; and the synthesized products have potential applications in biology, pharmacy and materials.
Owner:PEKING UNIV
Chemical synthesis method for azithromycin intermediate
The invention relates to a chemical synthesis method for an azithromycin intermediate. According to the chemical synthesis method for the azithromycin intermediate,erythromycin 6,9-imino ether is usedas a raw material and reduced by potassium borohydride and zinc trifluoromethanesulfonate, hydrolysis is assisted through IR.A-743 or ZXC-700 resin, and 9-deoxo-9-alpha-aza-9[alpha]-homoerythromycinA is obtained with a high yield.
Owner:QINGDAO AGRI UNIV
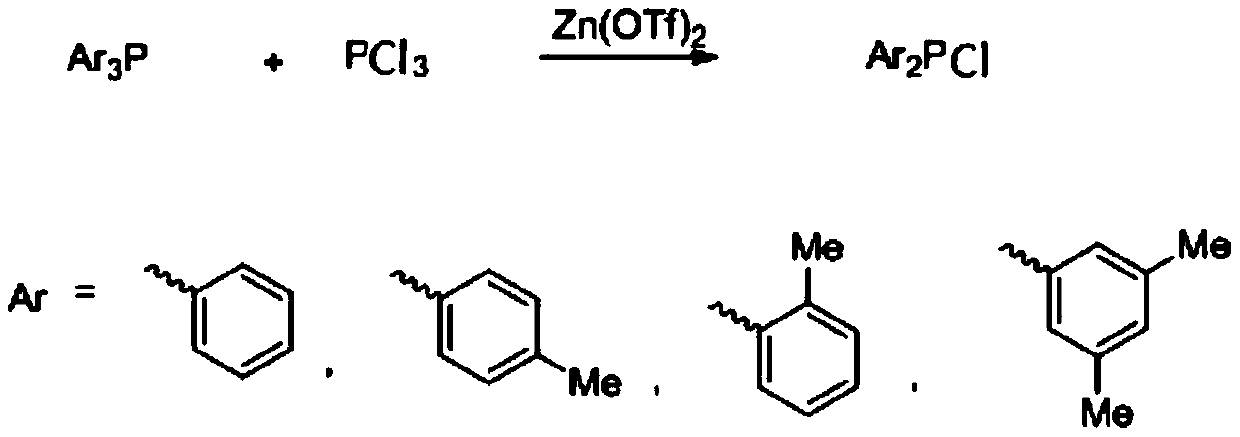
A kind of preparation method of diaryl phosphorus chloride compound
ActiveCN107936057BReduce usageMild reaction conditionsGroup 5/15 element organic compoundsDistillationMetal catalyst
The invention discloses a method for preparing a diarylphosphochlorine compound and belongs to the field of organic synthesis. The method is as follows: the diarylphosphochlorine compound is preparedby reaction of triarylphosphine as a starting material with phosphorus trichloride in the presence of zinc trifluoromethanesulfonate as a catalyst and distillation. Compared with the prior art, the method has the advantages of high reaction yield and simple post-treatment, is particularly suitable for preparation of the diarylphosphochlorine compound with a substituent, and is more suitable for industrial production. The obtained diarylphosphochlorine compound can be used as a ligand for synthesizing metal catalysts, and is applied to the fields such as organic photoelectric materials and medicines.
Owner:濮阳惠成新材料产业技术研究院有限公司
A kind of preparation method of organic porous material
ActiveCN109876778BImprove thermal stabilityGood chemical stabilityOther chemical processesDispersed particle separationPtru catalystThiourea
The invention provides a preparation method of an organic porous material. The preparation method includes the following steps that ion-like liquid and 2,4,6-trihydroxy-1,3,5-benzene trioxin are addedinto a reaction vessel, then a catalyst is added to be subjected to ultrasonic processing and mixing evenly, the reaction vessel is placed in liquid nitrogen to be frozen for 1-3 min, vacuumized to 0.01-1 pa, and sealed, and a product is obtained through a reaction for 24-72 h at 90-150 DEG C; the ion-like liquid is prepared from a hydrogen bond donor and a hydrogen bond receptor, and the hydrogen bond donor is one of carbamide and thiourea; the catalyst is one of scandium trifluoromethanesulfonate, europium trifluoromethanesulfonate, indium trifluoromethanesulfonate, ytterbium trifluoromethanesulfonate hydrate, yttrium trifluoromethanesulfonate, and zinc trifluoromethanesulfonate; the product is washed through a solvent, and filtered after Soxhlet extraction for 12-48 h; and the productis dried under the vacuum condition to obtain the organic porous material. According to the preparation method of the organic porous material, use of volatile and toxic organic solvents can be avoided, and environmental-friendly performance is improved.
Owner:CHINA UNIV OF MINING & TECH
Zinc trifluoromethanesulfonate-loaded spherical mesoporous material, and preparation method and application thereof
ActiveCN102039177BImprove conversion rateReduce pollutionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCyclohexanonePtru catalyst
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation methods of 2,2-dimethyl-1,3-dioxolane and ethylene glycol
ActiveCN103087035BEasy to useTo overcome the shortcomings of serious pollutionOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingIonic liquidZinc trifluoromethanesulfonate
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation methods of 2,2,4-trimethyl-1,3-dioxolane and 1,2-propanediol
ActiveCN103087034BEasy to useHigh catalytic efficiencyChemical recyclingPreparation by hydrolysisPropanediolIonic liquid
The invention provides a preparation method of 2,2,4-trimethyl-1,3-dioxolane. The method comprises the steps that: under a cyclization reaction condition, 1,2-propylene oxide and acetone contact a catalyst, such that a mixture is obtained; and the 2,2,4-trimethyl-1,3-dioxolane is separated from the mixture, wherein the catalyst comprises ionic liquid 1-alkyl-3-methylimidazolium salt and anhydrous zinc chloride. The invention also provides a preparation method of 1,2-propanediol. According to the 2,2,4-trimethyl-1,3-dioxolane preparation method provided by the invention, the catalyst composed of the ionic liquid 1-alkyl-3-methylimidazolium salt and anhydrous zinc chloride is adopted, such that defects such as high catalyst cost and severe environment pollution of prior arts in which zinc trifluoromethanesulfonate is adopted as a catalyst for catalyzing the reaction of 1,2-propylene oxide and acetone are solved. Also, catalytic efficiency is improved compared with a method for using single anhydrous zinc chloride as a catalyst to replace zinc trifluoromethanesulfonate, and catalyst recycling and reutilization can be facilitated.
Owner:CHINA PETROLEUM & CHEM CORP +1
A kind of synthetic method and application of imidazolone derivative
The invention provides synthesis method and application of an imidazole derivative. The method uses carbodiimide, hydrochloride salt of amino-acid ester, tertiary amine and zinc triflate in a catalytic amount to prepare 2-amino or imino substituted imidazole derivative. The synthesis method is scientific and rational, provides a general method for synthesis of an imidazole derivative with a variety of substituents, and can further synthesize more complex heterocyclic molecules, such as benzimidazole imidazolone. The method has the advantages of easily available raw materials, wide range of application, high separation yield, simple laboratory equipment and operations, is convenient for further development and application; and the synthesized products have potential applications in biology, pharmacy and materials.
Owner:PEKING UNIV
Preparation and application of zinc negative electrode material of novel aqueous zinc ion battery
PendingCN114361376AReduce direct contactIncrease contact areaElectrode manufacturing processesFinal product manufactureCorrosion reactionElectrical battery
The invention relates to preparation and application of a zinc negative electrode material of a novel aqueous zinc ion battery. The preparation method of the zinc negative electrode material comprises the following steps: dissolving polyvinyl chloride and zinc trifluoromethanesulfonate in a polar solvent at room temperature to form a precursor solution for preparing the electrode material; and blade-coating the precursor solution on the surface of a zinc metal substrate by using a simple blade-coating method to prepare the novel zinc negative electrode. A chlorine atom group in polyvinyl chloride can become an active site for guiding zinc deposition, uniform zinc deposition is induced, corrosion reaction is reduced, meanwhile, dendritic crystal formation can be inhibited, coulombic efficiency is improved, the electrochemical performance of a battery is improved, and the cycle life of the battery is prolonged.
Owner:QILU UNIV OF TECH
Catalyst prepared by loading zinc trifluoromethanesulfonate on macroporous-mesoporous material, and preparation method and use thereof
ActiveCN102039178BGood acid conversionEasy accessOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMesoporous materialPorous channel
The invention relates to a macroporous-mesoporous material SBA-15 loaded with zinc trifluoromethanesulfonate and a preparation method and use thereof. A novel catalyst is synthesized by loading zinc trifluoromethanesulfonate onto the mesoporous material SBA-15 with aperture between 10 and 14 nanometers. In the invention, macroporous-mesoporous material SBA-15 supported zinc trifluoromethanesulfonate is used as a catalyst for use in the methyl oleate synthesis reaction of oleic acid and methanol, the macropores in the catalyst allow macromolecular oleic acid and methanol to enter mesoporous porous channels to promote a catalytic reaction, side reactions in the catalysts reaction are reduced, product purity is improved, the catalyst can be recycled with high acid conversion rate and little pollution to the environment. The invention also provides a process for preparing a methyl oleate important chemical raw material by using the macroporous-mesoporous material to catalyze the reaction of oleic acid and methanol.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com