Aqueous zinc ion battery electrolyte for inhibiting vanadium dissolution of vanadium-based positive electrode as well as preparation method and application thereof
A zinc-ion battery, electrolyte technology, applied in water-based electrolytes, secondary batteries, acid electrolytes, etc., to achieve the effect of improving stability, reducing quantity, and inhibiting the activity of water molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of aqueous zinc-ion battery electrolyte:
[0027] On the basis of 2M zinc trifluoromethanesulfonate electrolyte, add 3M concentration of lithium trifluoromethanesulfonate, ultrasonically disperse for a certain period of time and dissolve to obtain an aqueous zinc-ion battery electrolyte.
[0028] Preparation of positive electrode sheet:
[0029] The active substance (VO 2 ), carbon black, and PVDF were formulated into a slurry in a ratio of 7:2:1, and after stirring for 24 hours, the slurry was coated on a stainless steel mesh, and vacuum-dried for 12 hours to obtain VO 2 Positive pole piece.
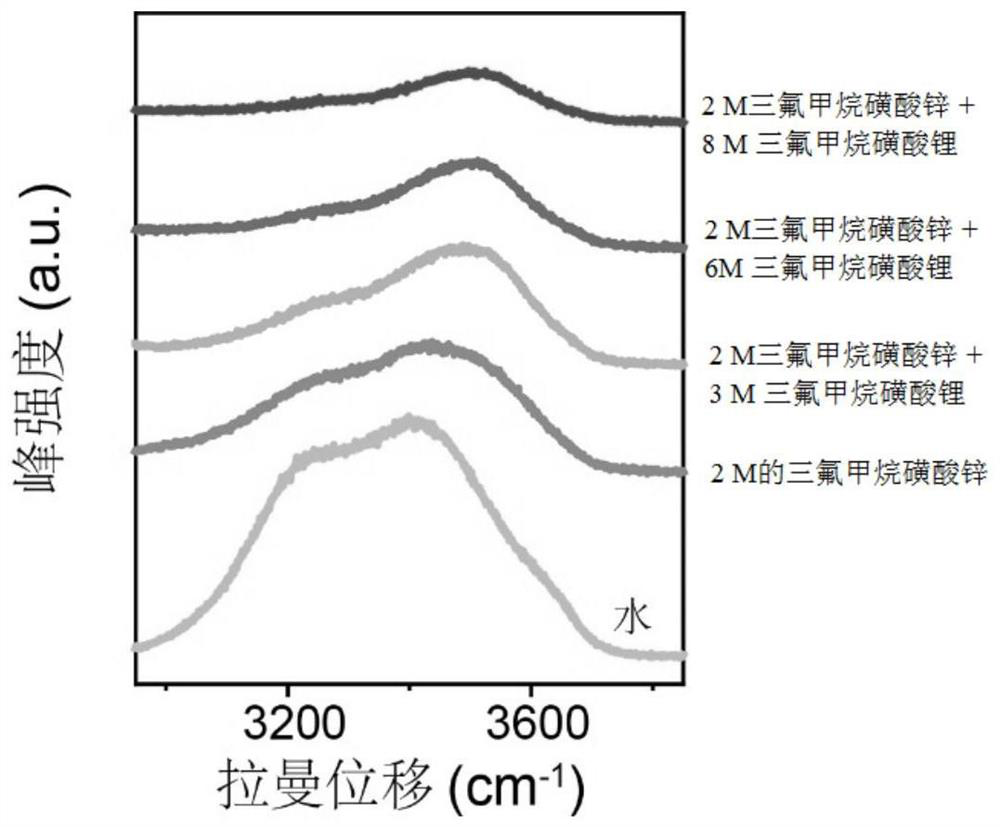
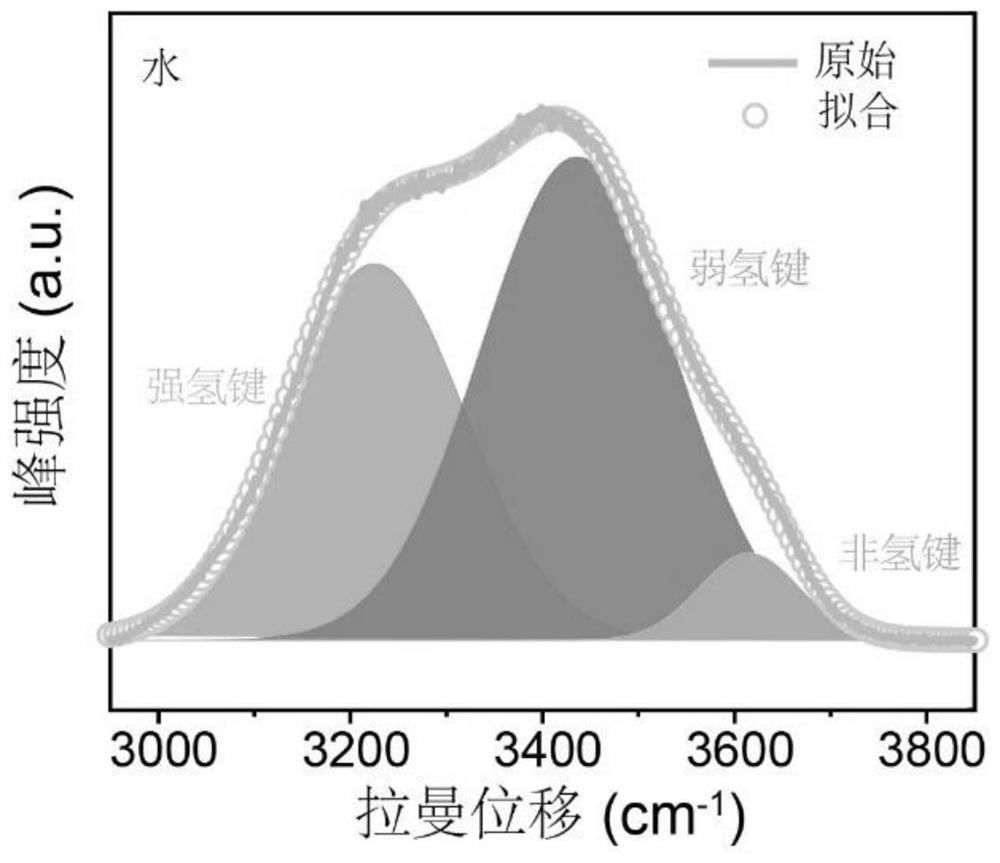
[0030] Use Raman (LabRAM HR, Horiba JobinYvon) to analyze the activity of water molecules in the electrolyte, and get figure 1 and figure 2 . From figure 1 and figure 2 It can be seen that as the concentration of lithium trifluoromethanesulfonate increases, the intensity of the water absorption peak corresponding to Raman decreases gradually, indicating that ...
Embodiment 2
[0035] On the basis of the 2M zinc trifluoromethanesulfonate electrolyte, add 6M lithium trifluoromethanesulfonate, ultrasonically disperse and dissolve for a certain period of time to obtain an aqueous zinc-ion battery electrolyte.
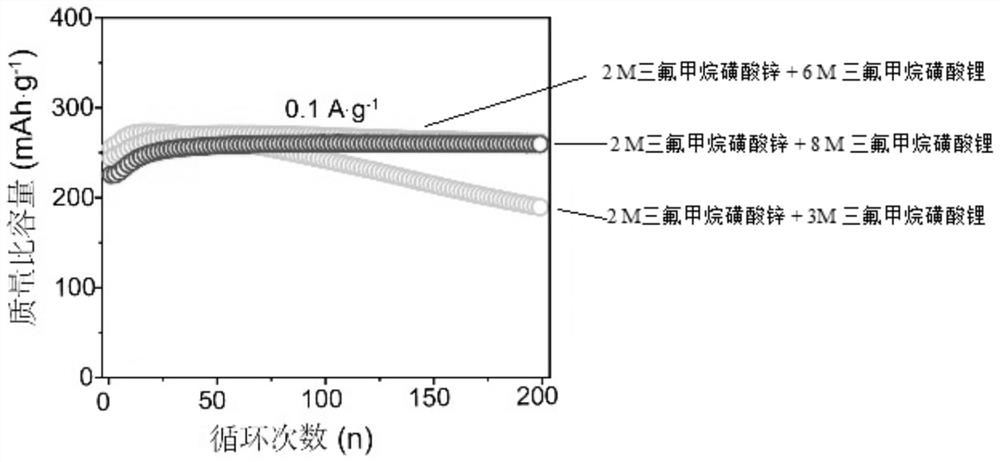
[0036] The positive electrode sheet and battery test conditions were the same as in Example 1. Test results such as image 3 , 4 As shown, it can be seen that the cycle stability of the battery is further improved.
Embodiment 3
[0038] On the basis of the 2M zinc trifluoromethanesulfonate electrolyte, add 8M lithium trifluoromethanesulfonate, ultrasonically disperse and dissolve for a certain period of time to obtain an aqueous zinc-ion battery electrolyte.
[0039] The positive electrode sheet and battery test conditions were the same as in Example 1. Test results such as image 3 , 4 As shown, it can be seen that the cycle stability of the battery has been greatly improved. On the premise of sacrificing part of the initial capacity, the higher the concentration, the better the cycle stability of the battery. Among them, 0.1A g at low current density -1After 200 cycles, its capacity retention rate still reaches 99.6%, basically no decline, and the high current density is 10A g -1 Next, after 10,000 cycles, the capacity retention rate is 89.7%. It shows that the invention has significantly improved battery stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com