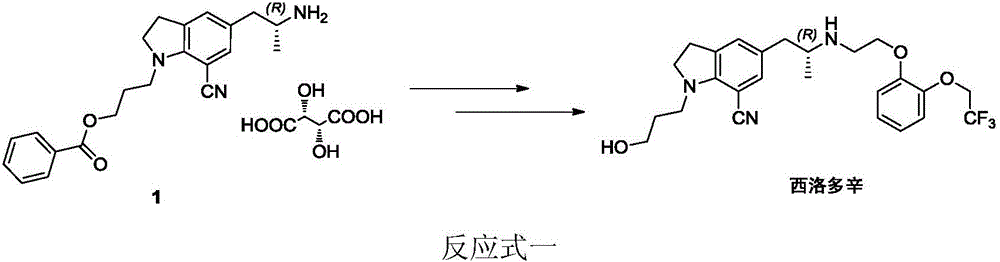
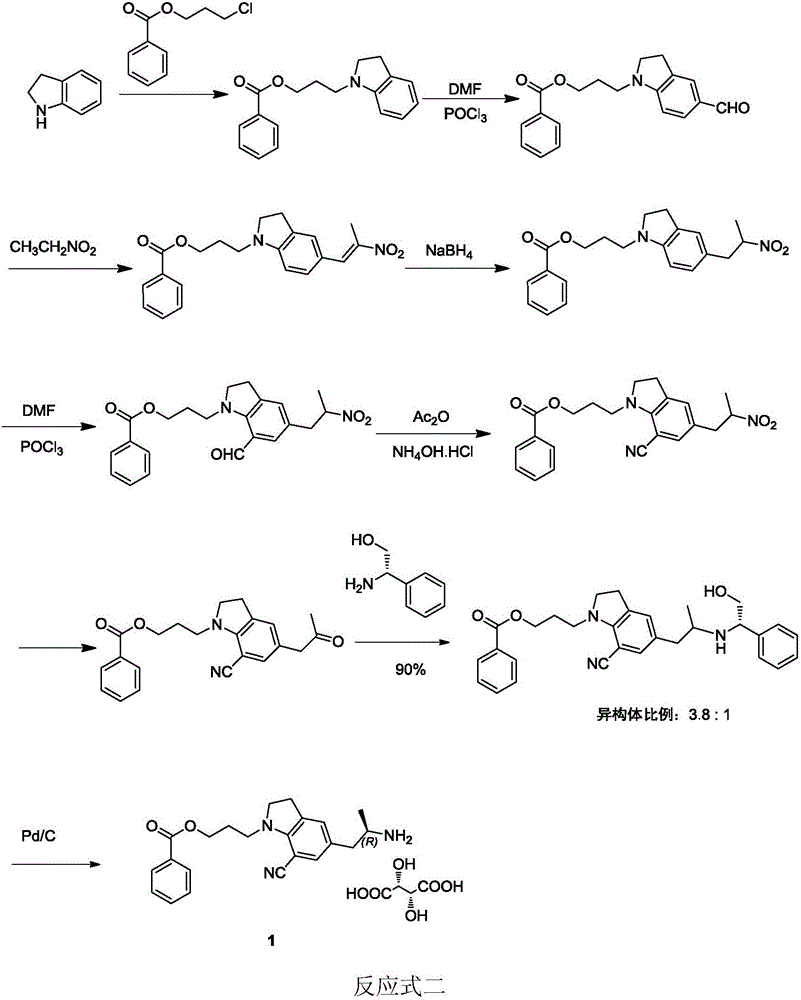
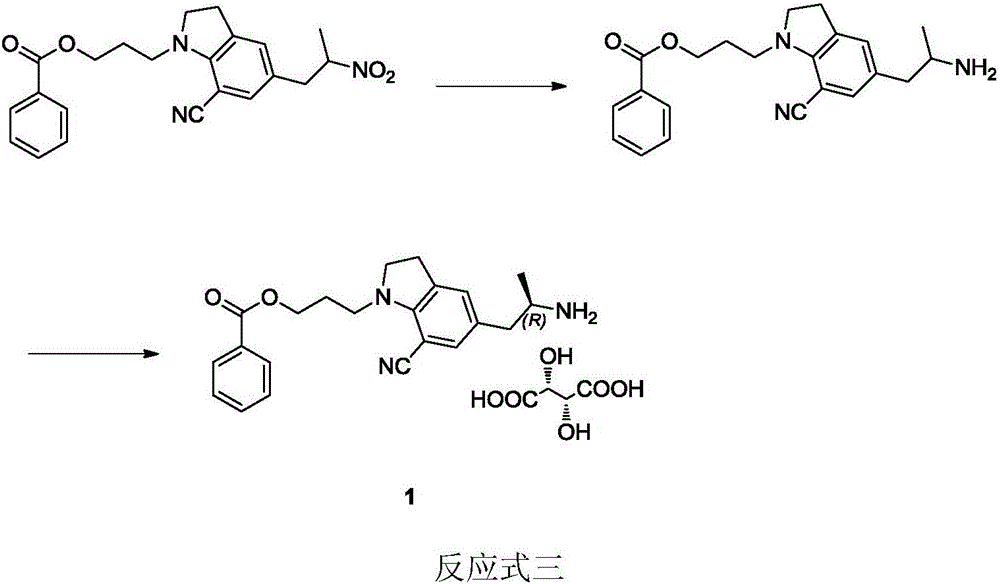
Preparation method of silodosin intermediate
A technology of silodosin and intermediates, applied in the field of drug synthesis, can solve the problems of difficult to obtain, expensive and difficult auxiliary materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of compound 4
[0084]
[0085] At -5-0°C, put 435g of zinc trifluoromethanesulfonate, 189g of (S)-2-chloropropionyl chloride, and 1500mL of 1,2-dichloroethane into the reaction vessel, and slowly add 306g of the compound dropwise at this temperature 2 in 200mL of 1,2-dichloroethane solution, keep it warm for 1 hour after dripping, then slowly raise the temperature to reflux reaction (83°C), monitor by liquid chromatography until the reaction is complete (8-24 hours), and cool the reaction solution to At room temperature, add cold 5% hydrochloric acid aqueous solution, wash the organic layer with sodium bicarbonate solution and saturated brine successively, collect the organic layer and dry it with anhydrous sodium sulfate, filter, and concentrate the filtrate to dryness under reduced pressure. Recrystallization from a mixed solvent of dichloromethane and methanol gave 317 g of light yellow solid 4, yield: 80%, melting point: 115°C.
[...
Embodiment 2
[0089] Example 2: Using (S)-2-bromopropionyl chloride instead of (S)-2-chloropropionyl chloride in Example 1, Compound 4 was prepared according to the method described in Example 1, with a yield of 84%.
[0090] NMR data is the same as in Example 1.
Embodiment 3
[0091] Embodiment 3: the preparation of compound 4
[0092]
[0093] At -5-0°C, put 787g of bismuth trifluoromethanesulfonate, 189g of (S)-2-chloropropionyl chloride, and 1500mL of 1,2-dichloroethane into the reaction vessel, and slowly add 306g of the compound dropwise at this temperature 2 in 200mL of 1,2-dichloroethane solution, keep it warm for 1 hour after dripping, then slowly raise the temperature to reflux reaction (83°C), monitor by liquid chromatography until the reaction is complete (8-24 hours), and cool the reaction solution to Add cold 5% hydrochloric acid aqueous solution at room temperature, wash the organic layer with sodium bicarbonate solution and saturated brine successively, collect the organic layer and dry it with anhydrous sodium sulfate, filter, and concentrate the filtrate to dryness under reduced pressure. The mixed solvent of methyl chloride and methanol was recrystallized to obtain 277g of light yellow solid 4, yield: 70%.
[0094] Nuclear magn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com