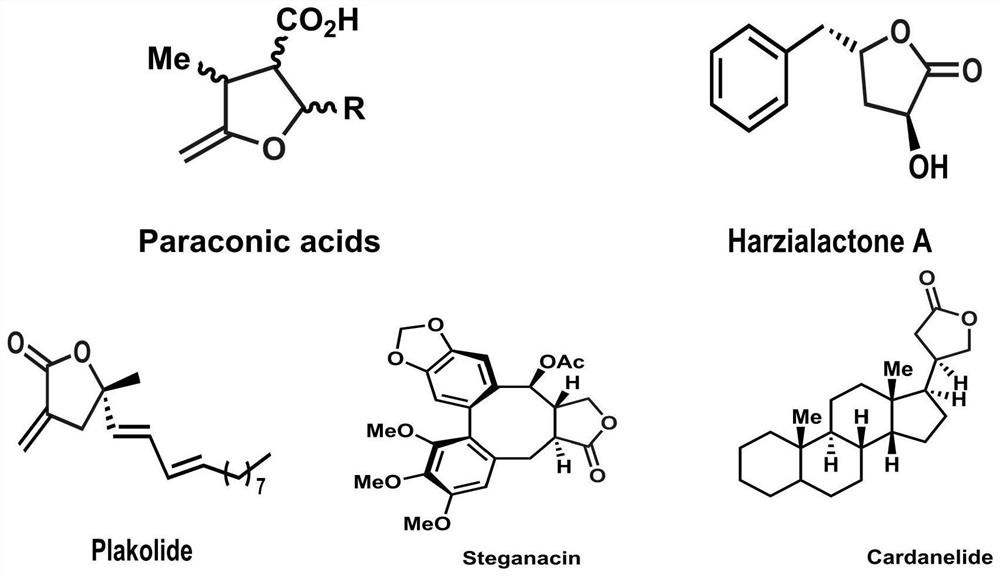
Method for asymmetrically synthesizing dihydrofuran-2-(3H)-one compound under catalysis of nickel
A technology of ketone compounds and dihydrofuran, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of unfriendly environment, high cost, high toxicity, etc., achieve high yield and enantioselectivity, low cost, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
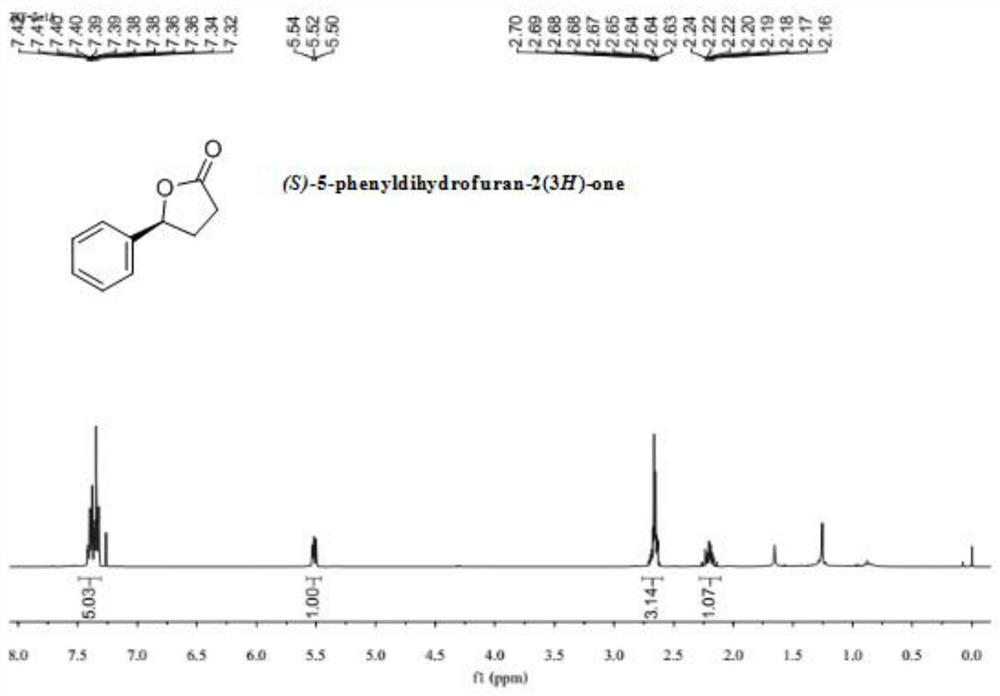
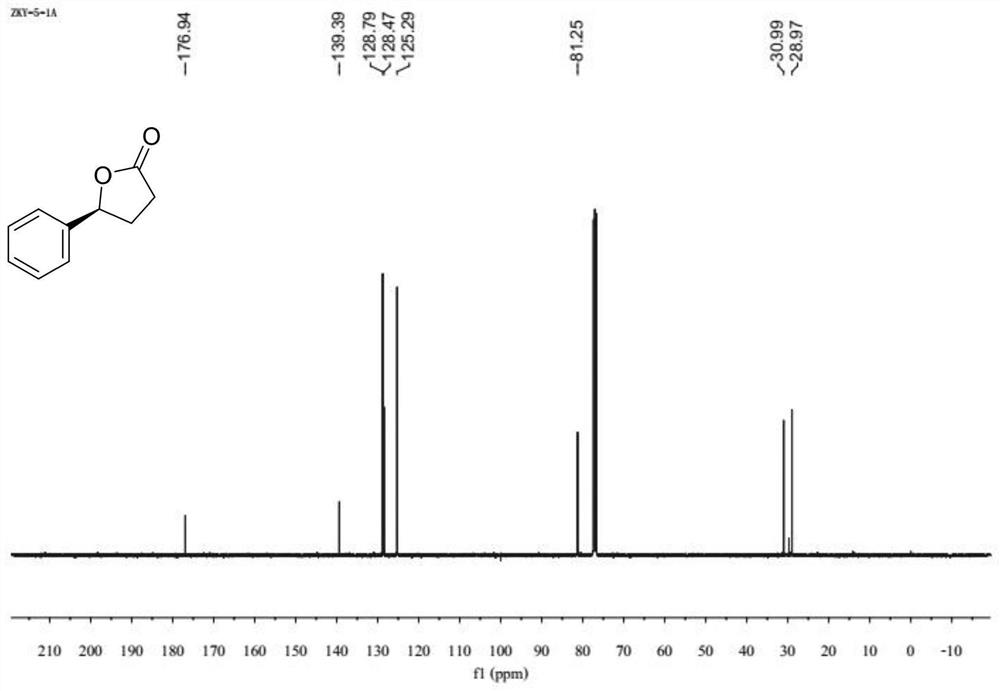
[0042] In the glove box, add accurately weighed 0.01 mmol of nickel acetate tetrahydrate and 0.012 mmol of the ligand into a Schneck reaction tube with a stirring bar, add 1 mL of 1,4-dioxane, and plug the rubber stopper Stir at room temperature for 30 min, then add 0.2 mmol of the substrate 3-benzoylpropionic acid, zinc trifluoromethanesulfonate (0.02 mmol) and 1 mL of 1,4-dioxane. Take out the reaction tube and put it into an autoclave, replace the hydrogen 3 times, the hydrogen pressure is 3 Mpa, set the temperature at 70°C under the oil bath, and wait until the reaction is complete. After the reaction was completed, the reaction tube was taken out, and the reaction liquid was removed under reduced pressure using a rotary evaporator to remove volatile solvents, and ethyl acetate / petroleum ether was used as an eluent, and purified by column chromatography to obtain the product. (96% yield, 94% ee). 1 H NMR (400MHz, CDCl 3 ) δ 7.49 – 7.30 (m, 5H), 5.58 – 5.46 (m...
Embodiment 2
[0044]
[0045] In the glove box, add accurately weighed 0.01 mmol of nickel acetate tetrahydrate and 0.012 mmol of the ligand into a Schneck reaction tube with a stirring bar, add 1 mL of 1,4-dioxane, and plug the rubber stopper Stir at room temperature for 30 min, then add 0.2 mmol of the substrate 3-benzoylpropionic acid, zinc trifluoromethanesulfonate (0.02 mmol) and 1 mL of 1,4-dioxane. Take out the reaction tube and put it into an autoclave, replace the hydrogen 3 times, the hydrogen pressure is 3 Mpa, set the temperature at 50°C under the oil bath, and wait until the reaction is complete. After the reaction was over, the reaction tube was taken out, and the reaction solution used a rotary evaporator to remove the volatile solvent under reduced pressure, and used ethyl acetate / petroleum ether as eluent, and purified by column chromatography to obtain the product (88% yield, 94% ee).
Embodiment 3
[0047]
[0048] In the glove box, add accurately weighed 0.01 mmol of nickel acetate tetrahydrate and 0.012 mmol of the ligand into a Schneck reaction tube with a stirring bar, add 1 mL of 1,4-dioxane, and plug the rubber stopper Stir at room temperature for 30 min, then add 0.2 mmol of the substrate 3-benzoylpropionic acid, zinc trifluoromethanesulfonate (0.02 mmol) and 1 mL of 1,4-dioxane. Take out the reaction tube and put it into an autoclave, replace the hydrogen 3 times, the hydrogen pressure is 3 Mpa, set the temperature at 90°C under the oil bath, and wait until the reaction is complete. After the reaction was over, the reaction tube was taken out, and the reaction solution used a rotary evaporator to remove the volatile solvent under reduced pressure, and used ethyl acetate / petroleum ether as eluent, purified by column chromatography to obtain the product (88% yield, 92% ee).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com