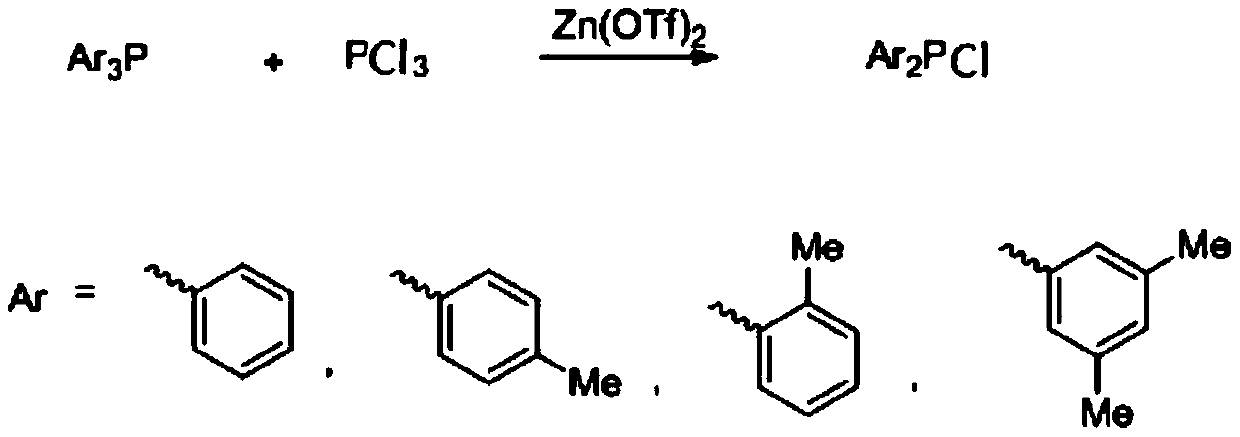
A kind of preparation method of diaryl phosphorus chloride compound
A technology for diarylphosphorus chloride and compound, which is applied in the field of organic synthesis, can solve the problems of limited application, complicated synthesis of diarylphosphorus oxygen compounds, etc., and achieves the effects of less waste and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0014] The synthesis of embodiment 1 diphenyl phosphorus chloride
[0015] Add triphenylphosphine (1mol, 262g), 1L carbon tetrachloride and zinc trifluoromethanesulfonate (0.01mol, 3.7g) into the dry reactor, and then add three Phosphorus chloride (0.5mol, 69g), after the dropwise addition, the temperature was raised to 60°C for 12 hours to stop the reaction. The reaction solution was filtered through diatomaceous earth, and the solvent was evaporated from the filtrate under normal pressure, and then diphenylphosphine chloride was distilled under reduced pressure to obtain 307g, with a yield of 93%; 31 P NMR (162MHz, CDCl 3 ),δ:80.5; 1 HNMR (400MHz, CDCl 3 ):7.65-7.68(m,4H),7.42-7.45(m,6H).
Embodiment 2 2
[0016] The synthesis of two (2-methylphenyl) phosphorus chlorides of embodiment 2
[0017] Add three (2-methylphenyl) phosphorus (1mol, 304g), 1L carbon tetrachloride and zinc trifluoromethanesulfonate (0.1mol, 36.2g) to the dry reactor, and then Phosphorus trichloride (0.5mol, 69g) was added dropwise to the system, and after the dropwise addition was completed, the temperature was raised to 80°C for 10 hours to stop the reaction. The reaction solution was filtered through diatomaceous earth, and the solvent was evaporated from the filtrate under normal pressure, and then distilled under reduced pressure to obtain 343 g of bis(2-methylphenyl)phosphorous chloride, with a yield of 92%; 31 P NMR (162MHz, CDCl 3 ):66.9; 1 HNMR (400MHz, CDCl 3 ):7.40-7.43(m,2H),7.21-7.25(m,2H),7.10-7.17(m,4H),2.41(s,6H).
Embodiment 3
[0018] The synthesis of two (4-methylphenyl) phosphorus chlorides of embodiment 3
[0019] Add three (4-methylphenyl) phosphorus (1.1mol, 334g), 1L carbon tetrachloride and zinc trifluoromethanesulfonate (0.05mol, 18.5g) to the dry reactor, and then Phosphorus trichloride (0.5mol, 69g) was added dropwise to the system, and after the dropwise addition was completed, the temperature was raised to 60°C for 12 hours to stop the reaction. The reaction solution was filtered through diatomaceous earth, and the solvent was evaporated from the filtrate under normal pressure, and then distilled under reduced pressure to obtain 339 g of bis(4-methylphenyl)phosphorous chloride, with a yield of 91%; 31 P NMR (162MHz, CDCl 3 ), δ: 81.6; 1 H NMR (400MHz, CDCl 3 ) 7.58 (d, J = 8.1 Hz, 4H), 7.34 (d, J = 8.1 Hz, 4H), 2.46 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com