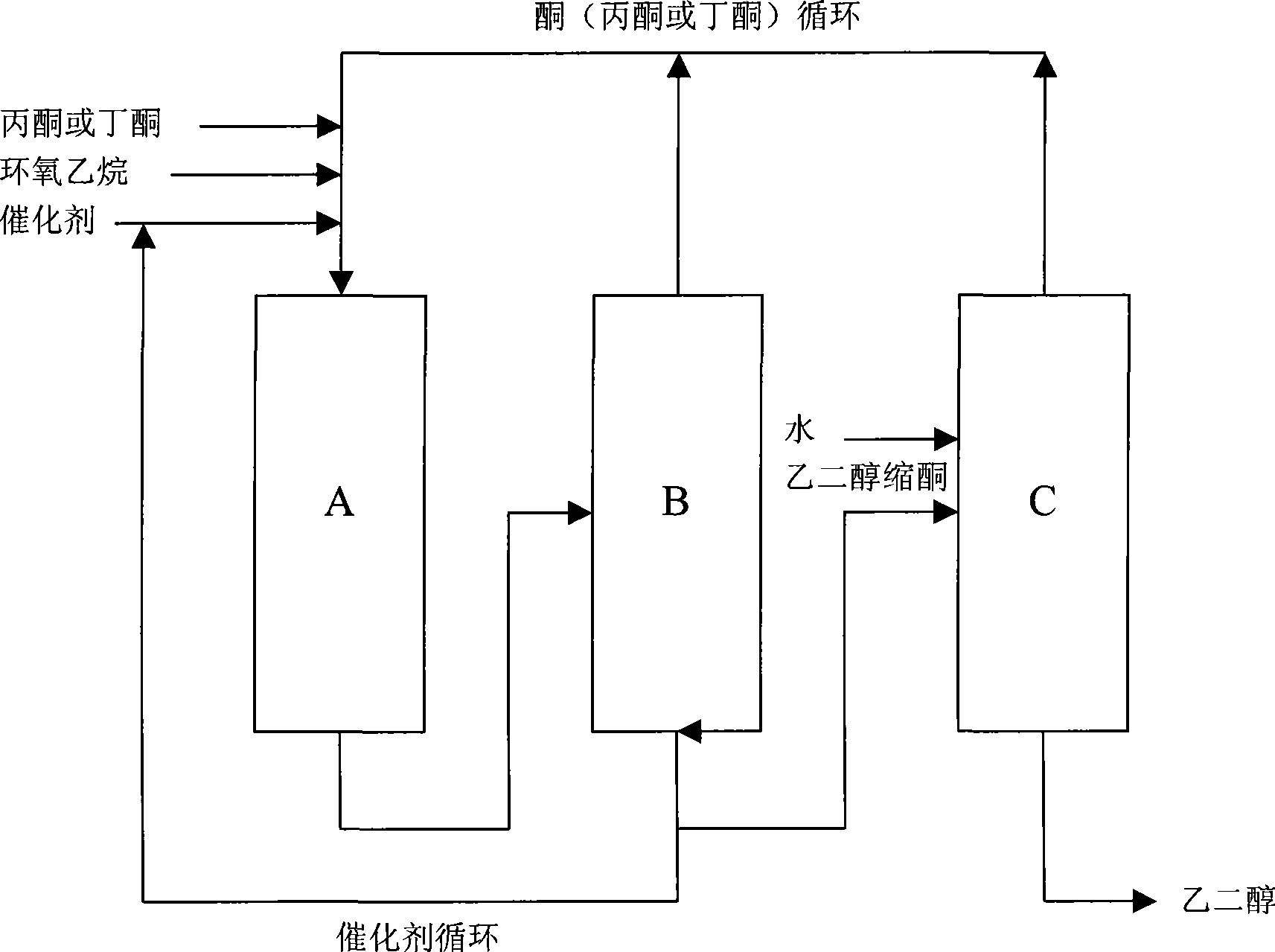
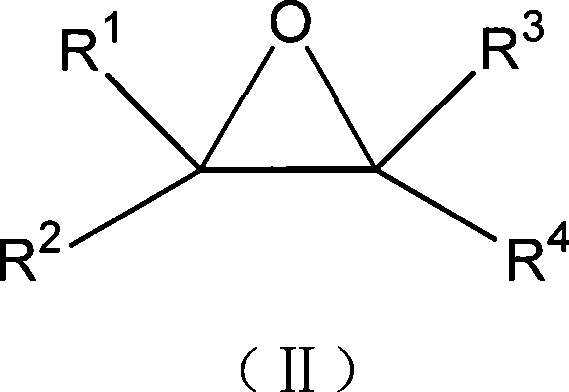
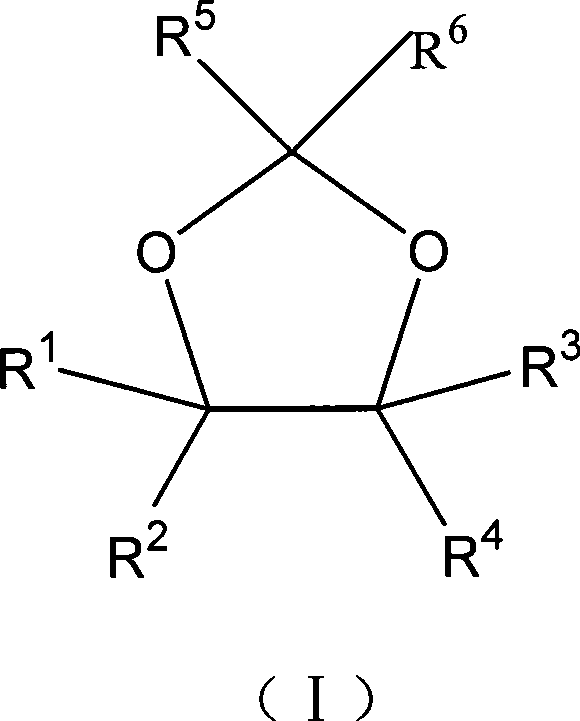
Method for preparing a 1,3-dioxolane compound
A technology of dioxolane and compound, applied in 1 field, can solve the problems of low ethylene glycol selectivity, high mass ratio, large energy consumption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: Preparation of 2,2-dimethyl-1,3-dioxolane
[0102] Add 21.2 grams of acetone and 4.4 grams of ethylene oxide into the 100ml polytetrafluoroethylene-lined reaction kettle, add 1.8 grams of zinc trifluoromethanesulfonate quickly, close the reaction kettle, and heat it in an oil bath at 55 ℃ magnetic stirring reaction for 8 hours, down to room temperature sampling analysis. The conversion rate is 98.77%, the selectivity is 99.06%, and the yield is 97.84%.
[0103] The composition of the reaction product liquid was analyzed by gas chromatography: the total content of by-products was 0.2%w. And it is 0.4%w in the document EP0448157B1.
Embodiment 2
[0104] Example 2: Preparation of 2,2-dimethyl-1,3-dioxolane
[0105] Add 11.6 grams of acetone and 4.4 grams of ethylene oxide into the reaction kettle with 100ml polytetrafluoroethylene lining, add 1.4 grams of zinc trifluoromethanesulfonate quickly, close the reaction kettle, heat in an oil bath, at 65 ℃ magnetic stirring reaction for 8 hours, down to room temperature sampling analysis. The conversion rate is 90.05%, the selectivity is 89.92%, and the yield is 80.97%.
Embodiment 3
[0106] Embodiment 3: Preparation of 2-methyl-2-ethyl-1,3-dioxolane
[0107] Add 28.8 grams of methyl ethyl ketone, 4.4 grams of ethylene oxide and 1.8 grams of zinc trifluoromethanesulfonate in a 100ml polytetrafluoroethylene-lined reaction kettle, close the reaction kettle, and react for 8 hours under heating in an oil bath at 55°C. Sample analysis at room temperature. The conversion rate is 99.71%, the selectivity is 96.69%, and the yield is 96.41%. Example 4: Preparation of 2-methyl-2-ethyl-1,3-dioxolane
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com