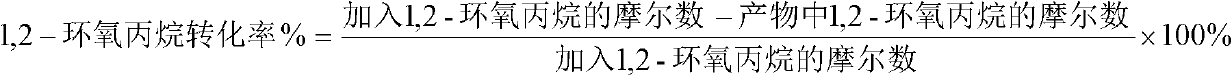Preparation methods of 2,2,4-trimethyl-1,3-dioxolane and 1,2-propanediol
A technology of dioxolane and trimethyl, applied in the field of preparation 2, can solve problems such as high price of zinc trifluoromethanesulfonate, environmental pollution, etc., and achieves the advantages of recycling and reuse, reducing energy consumption and cost, and improving catalysis. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
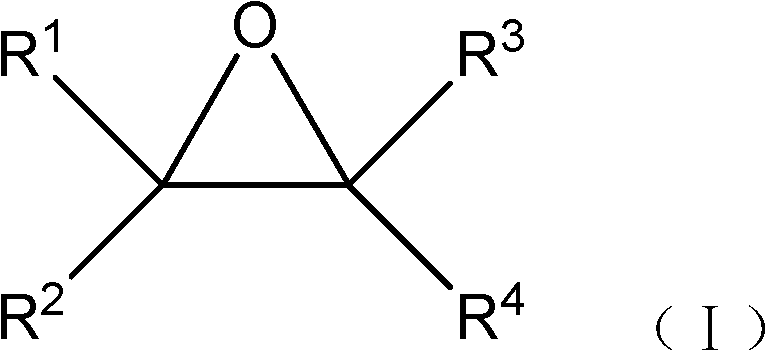
[0022] According to a preferred embodiment of the present invention, the cation of the ionic liquid 1-alkyl-3-methylimidazolium salt is 1-butyl-3-methylimidazolium, and the anion is bromide, chloride, trifluoro One of methanesulfonate ion, methanesulfonate ion, p-toluenesulfonate ion.
[0023] Specifically, the ionic liquid 1-alkyl-3-methylimidazolium salt can be 1-butyl-3-methylimidazole bromide, 1-butyl-3-methylimidazole chloride, 1-butyl -3-methylimidazolium bisulfate, 1-butyl-3-methylimidazolium methanesulfonate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate, 1-butyl-3-methyl imidazole p-toluenesulfonate, etc., more preferably the ionic liquid is 1-butyl-3-methylimidazole bromide. The ionic liquids mentioned above can be prepared by methods known in the art (see "Green Reaction Media in Organic Synthesis" edited by Koichi Mikami, 2005, Blackwell Publishing Ltd. et al.).
[0024] According to a preferred embodiment of the present invention, the ionic liquid 1-alky...
Embodiment 1
[0046] Put the magnetic stirring bar into a 25mL polytetrafluoroethylene-lined reactor, add 0.1mol (5.8g) acetone, 0.025mol (1.45g) 1,2-propylene oxide, and then add 0.01mol (2.19g) A catalyst composed of 1-butyl-3-methylimidazolium bromide ionic liquid and 0.005 mol (0.68 g) of anhydrous zinc chloride was uniformly mixed, and the reaction kettle was closed, and stirred and reacted for 8 hours in an oil bath at 65°C. After the reaction was completed, after cooling to room temperature, the liquid sample was taken for analysis. The conversion rate of 1,2-propylene oxide was 94%, and the selectivity of 2,2,4-trimethyl-1,3-dioxolane was 100%. %.
Embodiment 2
[0048] Put the magnetic stirring bar into a 100mL polytetrafluoroethylene-lined reaction kettle, add 0.2mol (11.6g) acetone, 0.05mol (2.9g) 1,2-propylene oxide, and then add 0.02mol brominated 1- Butyl-3-methylimidazolium ionic liquid and 0.01mol (1.36g) of anhydrous zinc chloride are uniformly mixed as a catalyst, and the reaction kettle is closed, and stirred and reacted for 12 hours in an oil bath at 55°C. After the reaction was completed, after cooling to room temperature, the liquid sample was taken for analysis. The conversion rate of 1,2-propylene oxide was 95%, and the selectivity of 2,2,4-trimethyl-1,3-dioxolane was 99.5%. %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com