Preparation method and application of metal organic cage compound
A cage compound and metal-organic technology, which is applied in the field of preparation of metal-organic cage compounds, can solve problems such as poor product quality, environmental hazards, and complicated processes, and achieve the effects of high yield, low price, and stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 4-amino-N-methylbenzamide (15.0g, 100mmol), methyl propiolate (16.8g, 200mmol) and benzaldehyde (10.6g, 100mmol) were added to 20mL of acetic acid, at 80°C Stir under the conditions for 1h, then cool the reaction solution and add it to 200mL of ice water, continue to stir for 1h, filter with suction, wash the filter cake with 300mL of ether, then recrystallize the filter cake with 100mL of ethanol to obtain a yellow powder (24.1g, yield 60%). 1 H-NMR (400MHz, CDCl 3 ):δ7.89(d,2H),7.74(s,2H),7.37(d,4H),7.31-7.27(m,1H),7.20(t,1H),6.21(d,1H),4.99( s,1H), 3.69(s,6H), 3.07(d,3H).
[0024] Weigh the yellow powder (12.18g, 30mmol) and mix with 150mL hydrazine hydrate, reflux and stir for 12h at 85°C, after the reaction, the product is suction filtered, and the filter cake obtained by suction filtration is washed with ethanol, and the filter cake after vacuum drying is dried. A white powder (5.79 g, 48% yield) was obtained. 1 H-NMR (400MHz, DMSO-d 6 ,ppm):δ9.17(s,2H),8.35(...
Embodiment 2
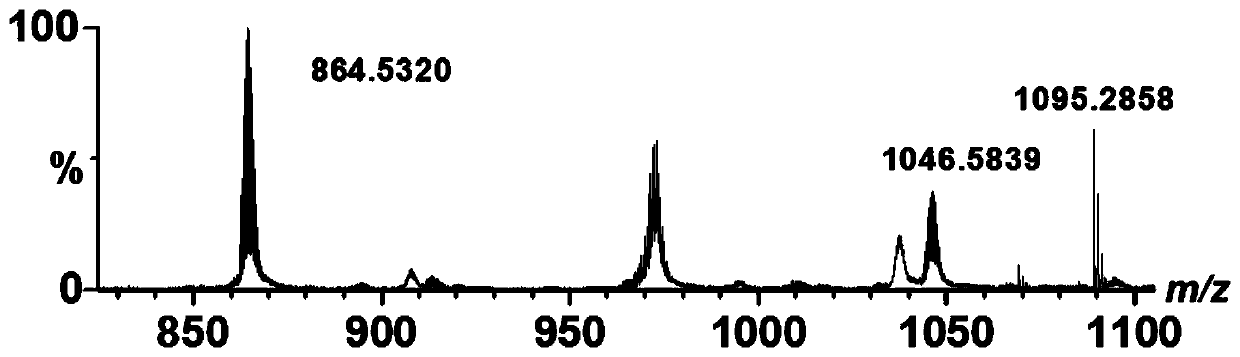
[0028] Weigh ligand H 2 ZPA (58.4mg, 0.1mmol) and Zn(NO 3 ) 2 ·6H 2 O (29.7mg, 0.1mmol) was added to 10mL of a mixed solvent of methanol and dichloromethane with a volume ratio of 1:1, stirred at room temperature for 4h, stirred and filtered, and the filtrate was left standing at room temperature for 2 weeks, and then the solution precipitated Yellow solid, the target compound Zn-ZPA (30.0 mg, yield 40%) can be obtained. Zn 4 C 132 h 112 N 32 o 12 ESI-MS:m / z:864.53[H 3 Zn 4 (ZPA) 4 ] 3+ ,1296.29[H 2 Zn 4 (ZPA) 4 ] 2+ .
Embodiment 3
[0030] Weigh ligand H 2 ZPA (58.4mg, 0.1mmol) and Zn(ClO 4 ) 2 ·6H 2 O (37.2mg, 0.1mmol) was added to 10mL of a mixed solvent of methanol and dichloromethane with a volume ratio of 1:1, stirred at room temperature for 4h, after stirring and filtering, the filtrate was left at room temperature for 2 weeks, and then precipitated out of the solution Yellow solid, the target compound Zn-ZPA (32.4 mg, yield 43.2%) can be obtained. Zn 4 C 132 h 112 N 32 o 12 ESI-MS:m / z:864.53[H 3 Zn 4 (ZPA) 4 ] 3+ ,1296.29[H 2 Zn 4 (ZPA) 4 ] 2+ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com