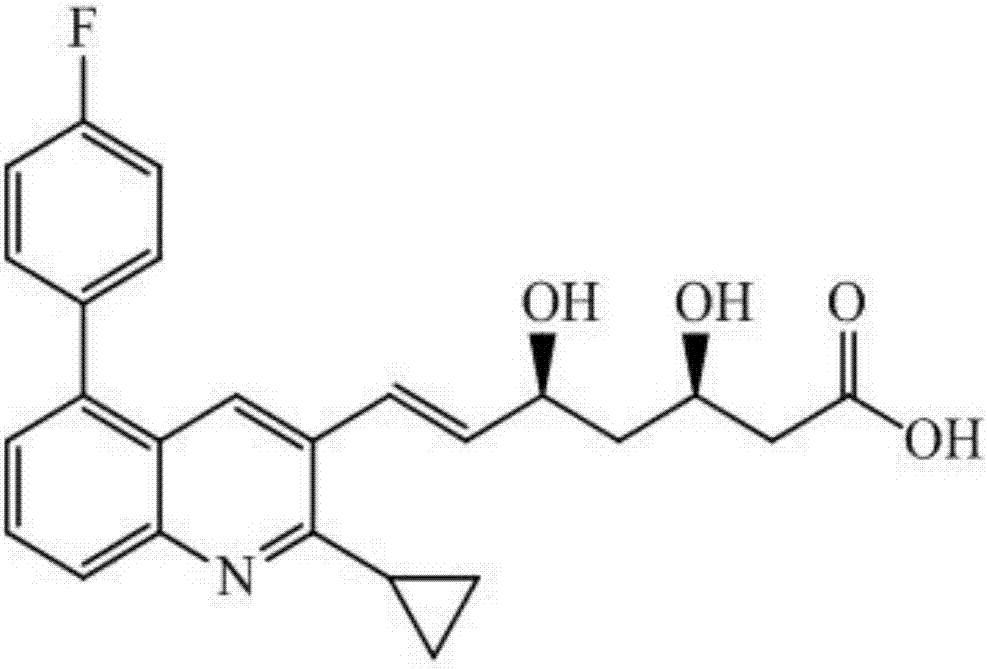
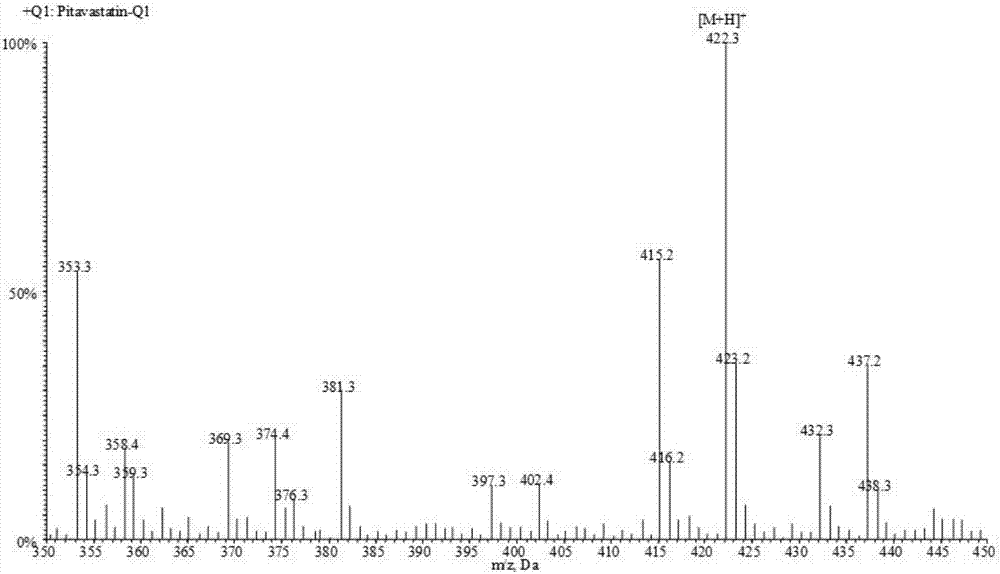
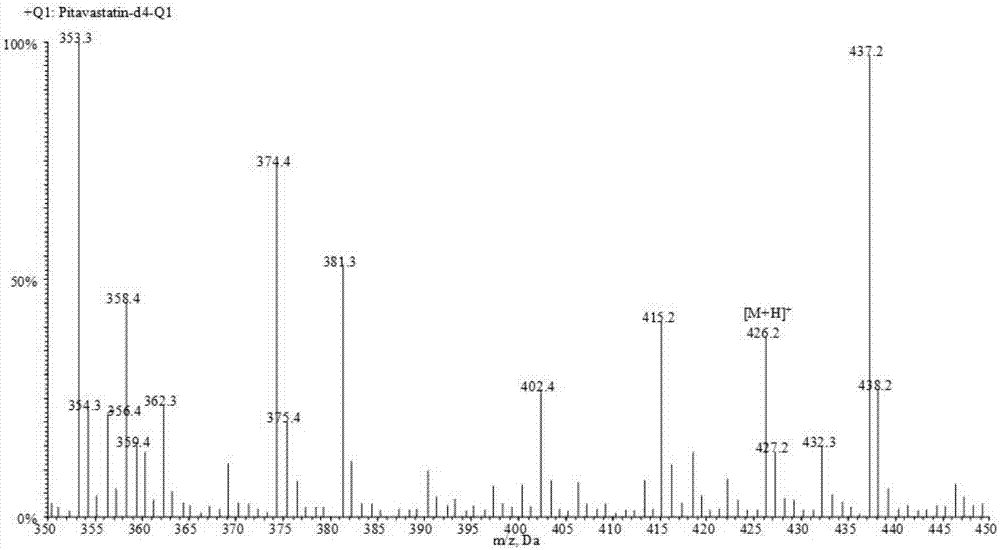
Liquid chromatogram-tandem mass spectrum method for detecting pitavastatin in human plasma, and application to clinical pharmacokinetic research
A technology of pitavastatin and liquid chromatography, which is applied in the field of liquid chromatography-tandem mass spectrometry for the detection of pitavastatin in human plasma, can solve the problems of complex pretreatment, drug instability, and long analysis period of biological samples, and achieve detection Fast, less interference, meet the detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0051] Abbreviation
[0052]
[0053] 1 material
[0054] 1.1 Instrument
[0055] Chromatograph: LC-30AD fast liquid chromatography system, Shimadzu Corporation, Japan.
[0056] Mass spectrometer: Model 6500 triple quadrupole tandem mass spectrometer equipped with an electrospray ionization source (Turbo Ion Spray) from Sciex, Canada.
[0057] Software used for data processing: Analyst (version 1.6.3), Sciex, Canada.
[0058] Centrifuge: HerμLe Z2326K desktop centrifuge, Germany Hermer Company.
[0059] Analytical balance: CD225D analytical balance, Beijing Sartorius Instrument Co., Ltd.
[0060] 1.2 Reference substances and reagents
[0061] Pitavastatin calcium (purity, 88.2%) was provided by Shenzhen Xinlitai Pharmaceutical Co., Ltd., d 4 - Pitavastatin calcium (purity, 98.6%) was purchased from Toronto Research Chemicals, Canada. Methanol, acetonitrile, and ammonium acetate (HPLC grade) were purchased from Sigma, USA. Deionized water (18.2mΩ, TOC≤50ppb) was prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com