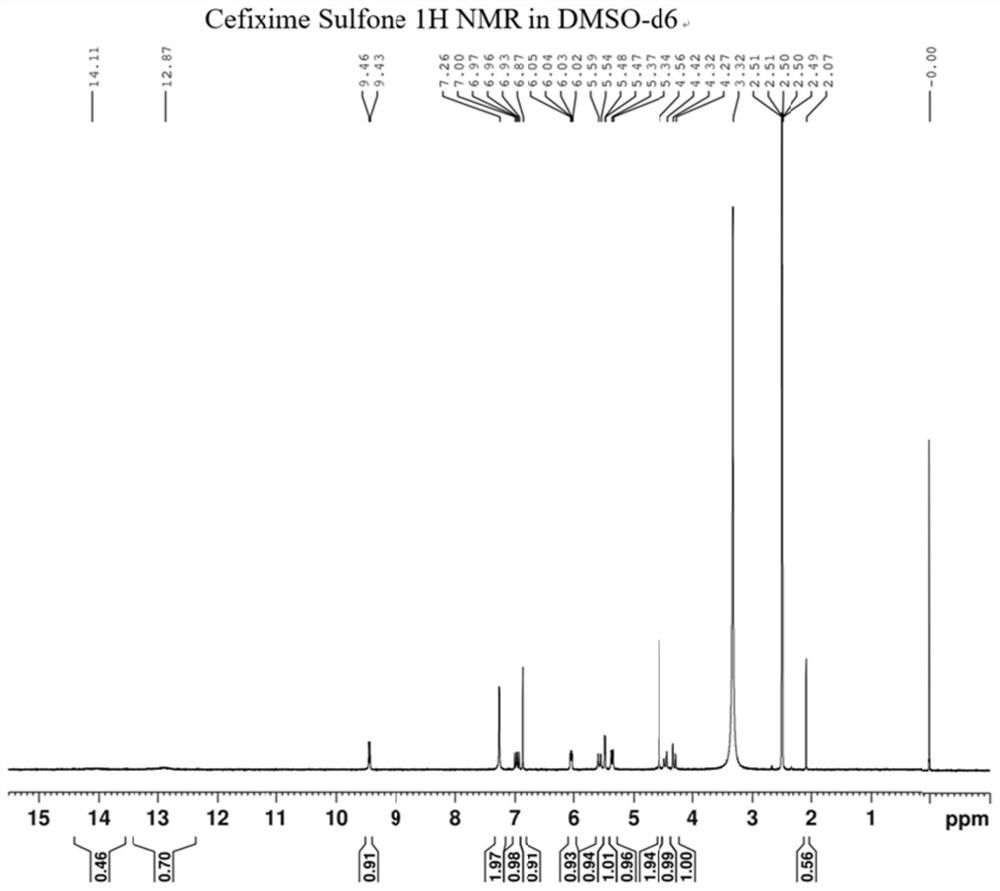
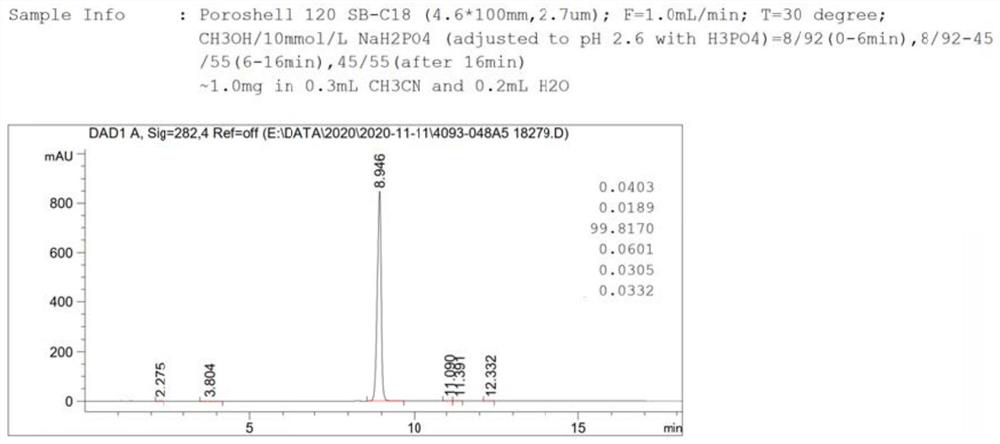
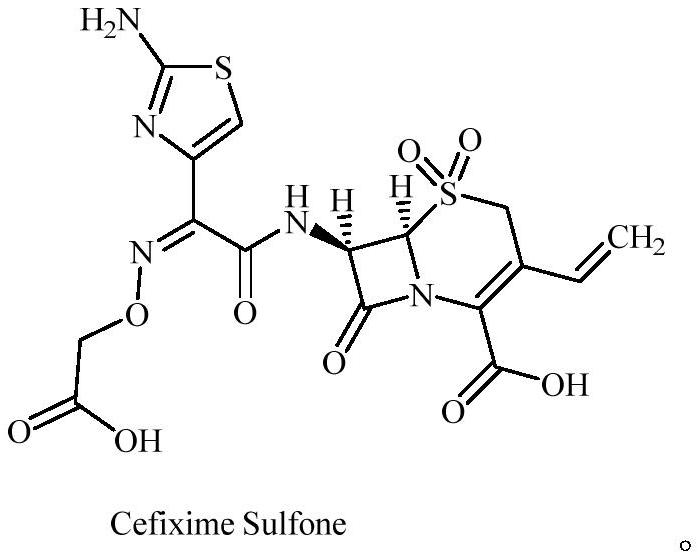
A kind of cefixime impurity and preparation method thereof
A technology for cefixime and impurities, applied in the field of cefixime impurities and its preparation, to achieve the effects of reasonable route design, simple post-treatment, and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of compound A: Suspend 30 g of cefixime in 300 mL of tert-butyl propionate, add 16.4 mL of boron trifluoride ether in an ice bath, and react at 40 degrees for 8 hours. Dry over anhydrous sodium sulfate, filter, spin dry, and purify the crude product through column to obtain 27.8 g of white solid A with a yield of 74.3%.
[0033]
[0034] Preparation of compound B: Dissolve 15.2 g of compound A in 304 mL of acetonitrile, then add 18.3 g of 25% hydrogen peroxide and 0.1 g of sodium tungstate, react at 10 degrees Celsius for 12 hours, monitor the reaction by TLC, dilute with 600 mL of ethyl acetate, wash with water three times, Dry over anhydrous sodium sulfate, filter, spin dry, and purify by column to obtain 10.2 g of white solid B with a yield of 63.5%.
[0035]
[0036] Preparation of compound Cefixime Sulfone: Dissolve 10 g of compound B in 30 mL of formic acid, react at 30 degrees Celsius for 10 hours, monitor the reaction by TLC, spin dry the react...
Embodiment 2
[0039] The preparation of compound A: 30g of cefixime was suspended in 600mL of tert-butyl propionate, 19.8mL of concentrated sulfuric acid was slowly added dropwise in an ice bath, and the reaction was performed at 25 degrees Celsius for 6 hours. Dry over sodium sulfate, filter, spin dry, and purify the crude product by column to obtain 29.5 g of white solid A with a yield of 78.8%.
[0040]
[0041] The preparation of compound B: get 15.5g compound A and dissolve in 310mL acetonitrile, then add 16.7g peracetic acid, 30 degrees Celsius of reaction 8 hours, TLC monitoring reaction finishes, add 100mL saturated sodium thiosulfate aqueous solution, quench excess oxidant, The organic phase was washed three times with water, dried over anhydrous sodium sulfate, filtered, spin-dried, and purified by column to obtain 8.3 g of white solid B with a yield of 50.7%.
[0042]
[0043] The preparation of compound Cefixime Sulfone: get 8.0g compound B and be suspended in 80mL 2M hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com