Method for detecting florfenicol and metabolite florfenicol amine in pig urine
A technology of florfenicol and florfenicol, which is applied in the field of veterinary drug residue detection, can solve problems such as increasing the difficulty of processing procedures, and achieve the effects of reliable quantitative analysis results, good specificity, and reduced detection costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
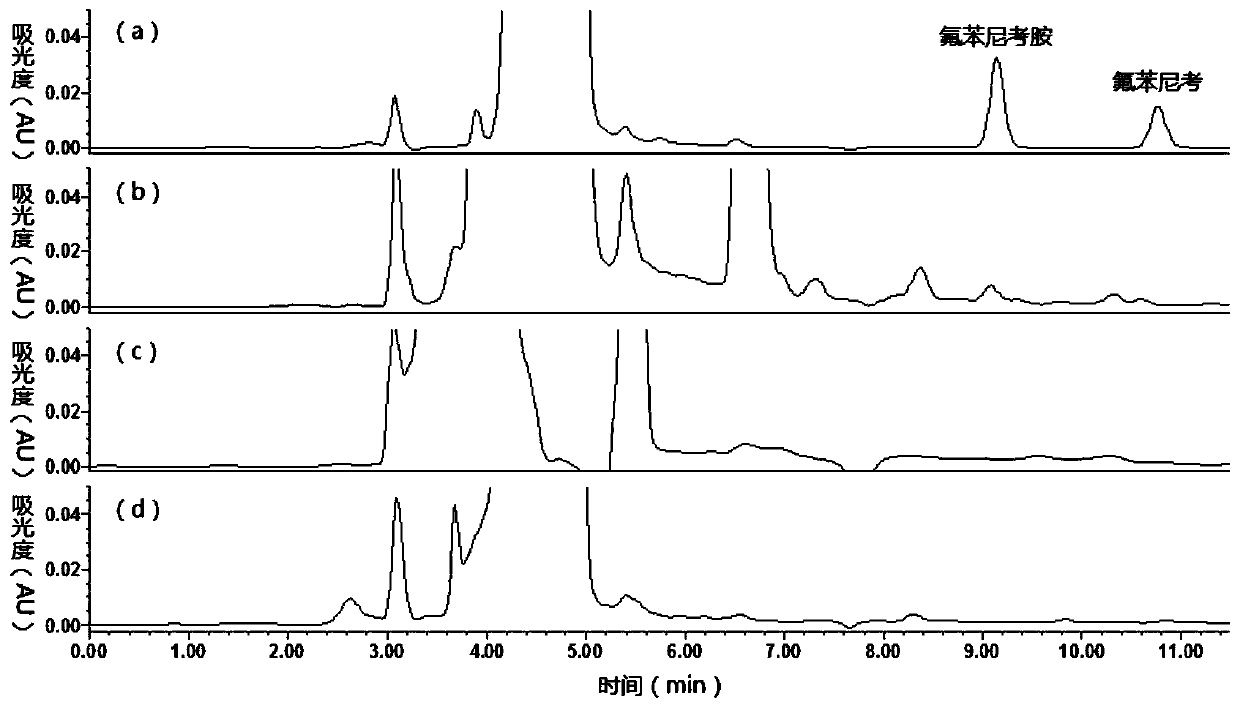
[0042] The comparison of various sample purification techniques purification effect of embodiment 1
[0043] 1. Method
[0044] (1) Accurately measure 2 mL of fresh blank pig urine, add 0.06 mL of 2.5N sodium hydroxide solution, and vortex for 1 min.
[0045] (2) Add 8 mL of ethyl acetate to the alkalized urine, vortex for 2 min, centrifuge at 5000 g for 5 min, and transfer the ethyl acetate to another clean centrifuge tube.
[0046] (3) Repeat the above operation once with an equal amount of ethyl acetate, and combine the ethyl acetate.
[0047] (4) Transfer ethyl acetate in batches to centrifuge tubes containing 0.6 mL of 5% glacial acetic acid solution, and evaporate the ethyl acetate to dryness with air flow at 50°C.
[0048] (5) The remaining glacial acetic acid solution was treated in the following three ways (repeat 5 samples for each method): ① without purification, dry the glacial acetic acid solution in air flow at 45°C, add 1 mL of mobile phase to redissolve the r...
Embodiment 2
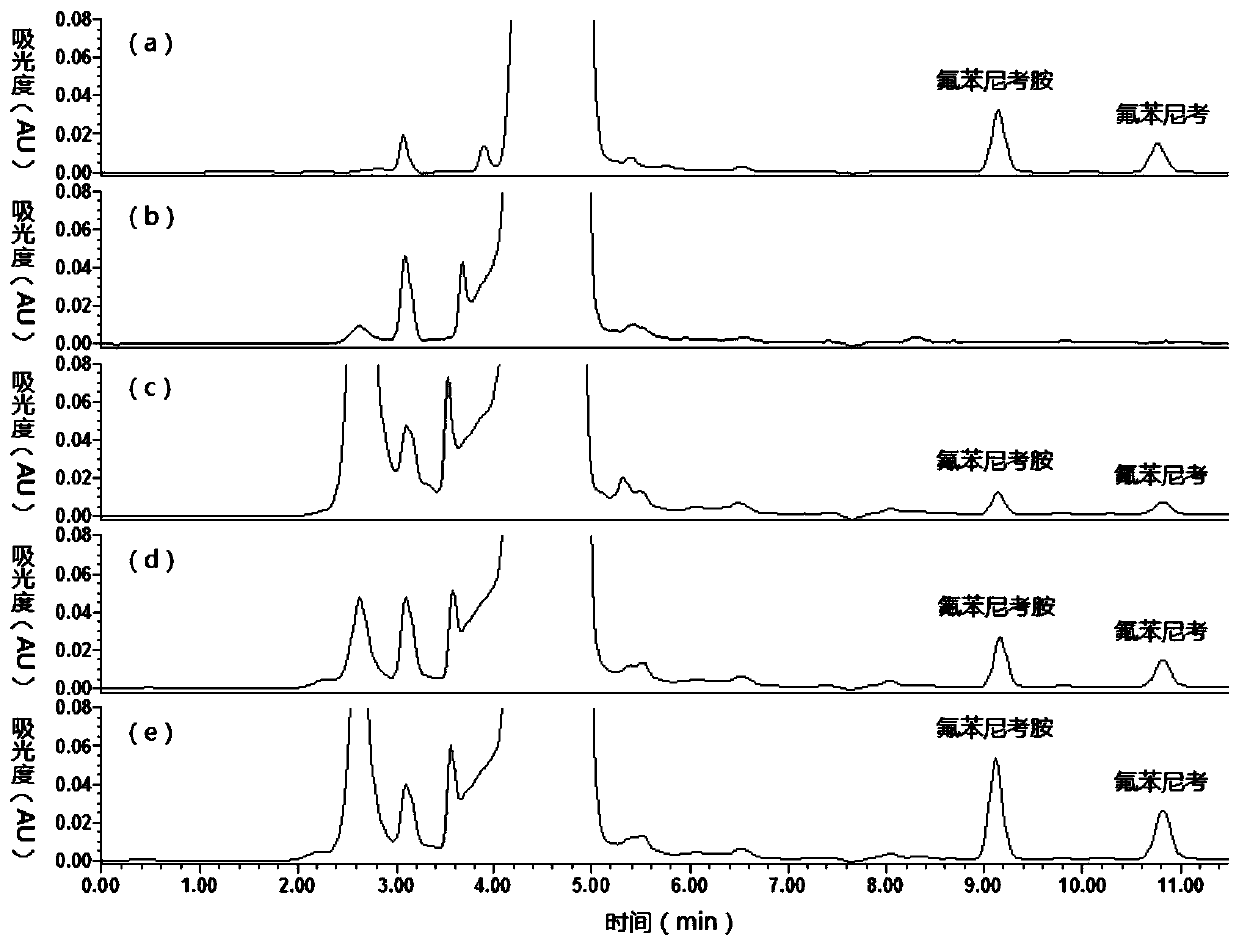
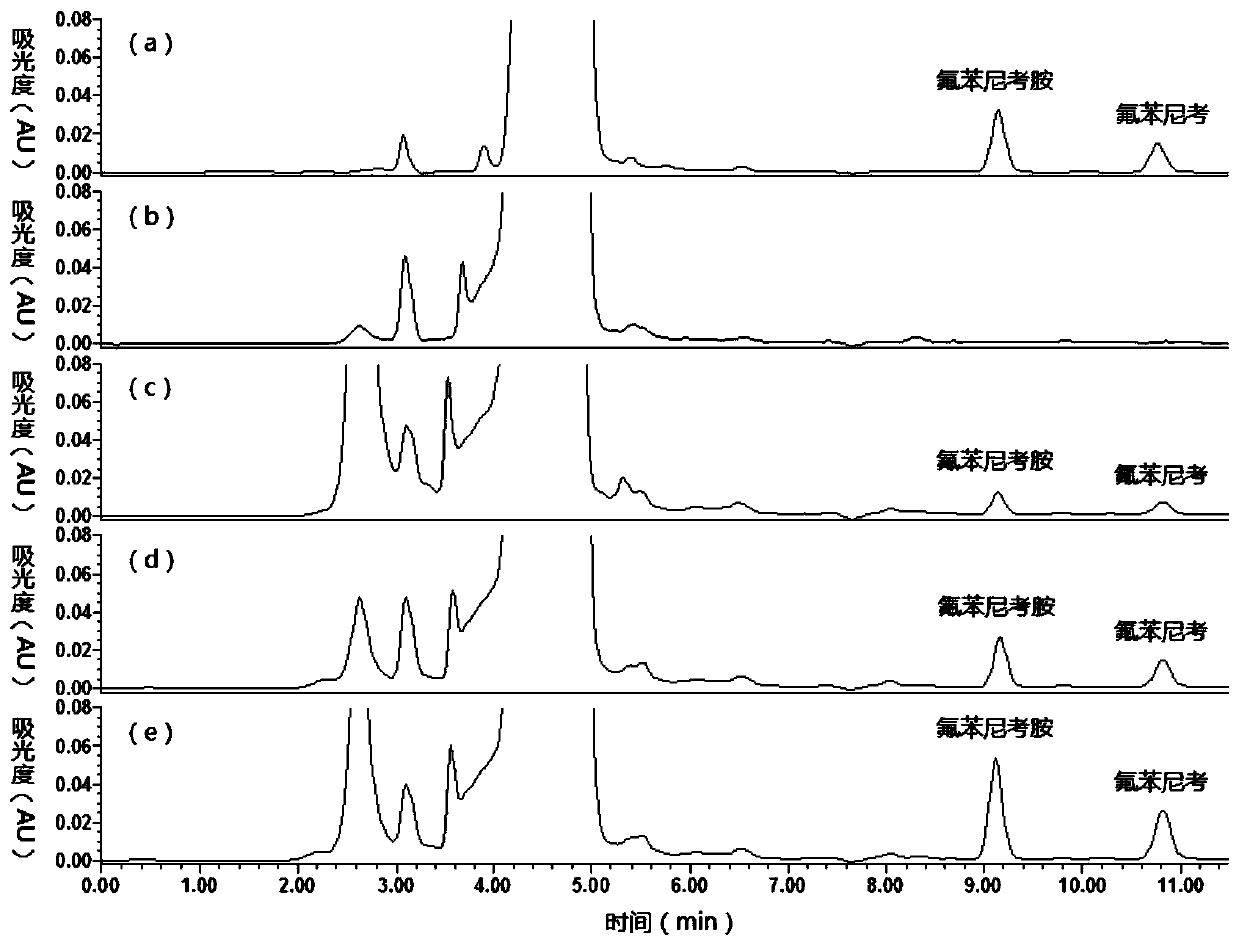
[0052] The accuracy and precision test of florfenicol and florfenicol amine in the pig urine of embodiment 2
[0053] 1. Method
[0054] (1) Accurately measure 2 mL of pig urine, add 0.25, 0.5 and 1 μg / mL florfenicol and florfenicol amine to it, repeat 5 samples for each concentration, and repeat three batches continuously.
[0055] (3) Process the above sample according to the extraction, concentration and thin-layer chromatography purification procedures shown in Example 1, and analyze according to the chromatographic conditions shown in Example 1. Florfenicol and florfenicol amine are quantified using a standard curve, and a series of concentration florfenicol amine standard products are detected by high performance liquid chromatography, with the concentration of florfenicol amine as the abscissa and the peak area as the ordinate, Obtain the regression equation y=143064x-1045.3 of Florfenicol and the regression equation y=275826x+1888.8 of Florfenicol amine, wherein x is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com