Heterocyclic compound and application thereof
A technology of heterocyclic compounds and heteroatoms, applied in the field of heterocyclic compounds, can solve the problems of single structure of TRPC5 inhibitors, and achieve good clinical pharmacokinetic properties, good metabolic stability, and good inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
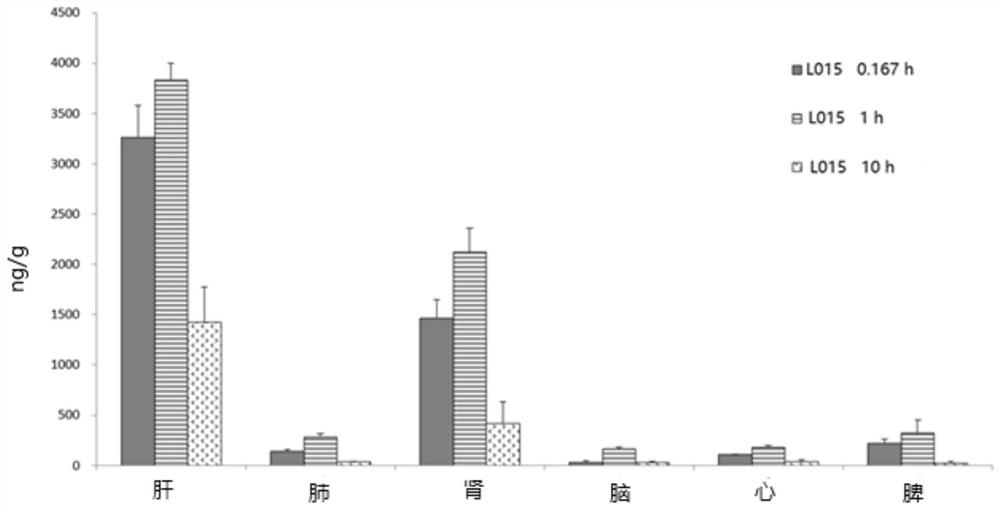
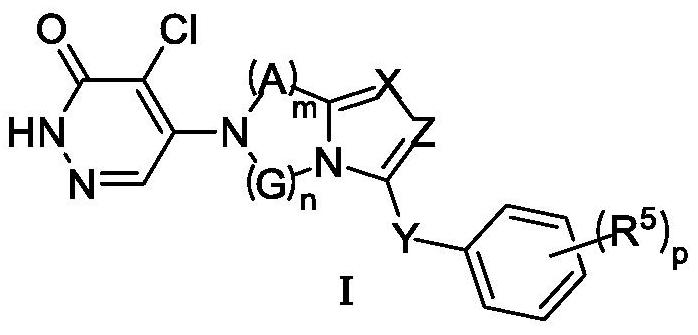
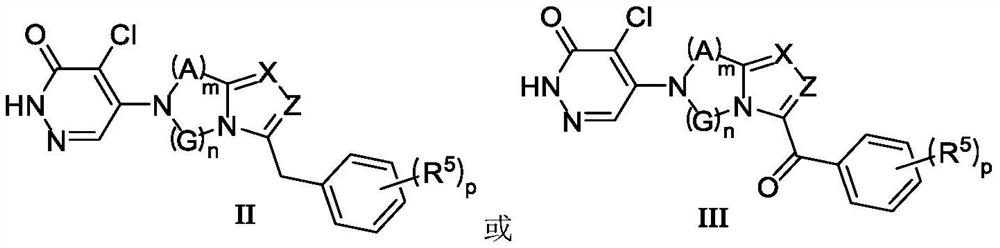
Image
Examples
Embodiment 1
[0486] Example 1 Compound 4-chloro-5-(3-(2-(trifluoromethyl)benzyl)-5,6-dihydroimidazo[1,2-a]pyrazin-7(8H)-yl ) Preparation of pyridazin-3 (2H) ketone (L001)
[0487]
[0488] 1.1 Preparation of compound 5,6-dihydroimidazo[1,2-a]pyrazine-7(8H)-tert-butyl carboxylate (L001-1)
[0489] 5,6,7,8-tetrahydroimidazo[1,2-a]pyrazine (700mg, 5.68mmol) and K 2 CO 3 (1.57g, 11.37mmol) add in the one-necked bottle, add H 2 O (8mL) and THF (8mL), after the complete dissolution, add dropwise (Boc) 2 O, react overnight at room temperature after the addition is complete. TLC (DCM / MeOH=10 / 1) showed that the reaction was complete. NaCl was added to the reaction solution to make it saturated, extracted with EA, and the organic phase was dried over anhydrous sodium sulfate and concentrated to obtain compound L001-1 (1.2 g) as a brown solid.
[0490] 1.2 Preparation of compound 3-bromo-5,6-dihydroimidazo[1,2-a]pyrazine-7(8H)-tert-butyl carboxylate (L001-2)
[0491] Add tert-butyl 5,6-dihy...
Embodiment 2
[0500] Example 2 Compound 5-(2-bromo-3-(4-fluoro-2-(trifluoromethyl)benzyl)-5,6-dihydroimidazo[1,2-a]pyrazine-7 (8H)-yl)-4-chloropyridazin-3(2H)-one (L002) preparation process
[0501]
[0502]
[0503] 2.1 Preparation of compound imidazo[1,2-a]pyrazine-3-carbaldehyde (L002-1)
[0504]Aminopyrazine (10.0 g, 0.11 mol) was dissolved in absolute ethanol (100 mL), 2-bromomalondialdehyde (15.8 g, 0.11 mmol) was added, and the mixture was stirred at 90° C. for 2 h. After the reaction, cool to room temperature, spin the solvent to obtain a black viscous object, add 30mL of hydrogen chloride in 1,4-dioxane solution (4mmol / L), stir at 25°C for 12h, after the reaction, add 50mL of water, Use sodium carbonate solution to adjust the pH value to about 8, extract with ethyl acetate (50mL*4), wash the organic phase with saturated brine (30mL*2), dry with anhydrous sodium sulfate, spin dry the solvent, and reconstitute with ethyl acetate After three crystallizations, 3.5 g of a yellow...
Embodiment 3
[0523] Example 3 Compound 7-(5-chloro-6-carbonyl-1,6-dihydropyridazin-4-yl)-3-(4-fluoro-2-(trifluoromethyl)benzyl)-N Preparation of -methyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyrazine-2-carboxamide (L003)
[0524]
[0525] 3.1 Compound 3-(4-fluoro-2-(trifluoromethyl)benzyl)-2-iodo-5,6-dihydroimidazo[1,2-a]pyrazine-7(8H)-carboxylate Preparation of tert-butyl acid ester (L003-1)
[0526] 3-(4-fluoro-2-(trifluoromethyl)benzyl)-5,6-dihydroimidazo[1,2-a]pyrazine-7(8H)-carboxylic acid tert-butyl ester ( L002-6, 0.50 g, 1.25 mmol) was dissolved in dichloroethane (6 mL), and N-iodosuccinimide (0.42 g, 1.87 mmol) was added. Under the protection of nitrogen, the reaction solution was stirred at 90° C. for 1 hour. After the reaction, cool to room temperature, add sodium thiosulfate aqueous solution (10mL) and stir for five minutes, extract with dichloromethane (10mL*3), dry over anhydrous sodium sulfate, filter, and the crude product after the filtrate is concentrated is subjected to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com