Method for synthesizing dl-norepinephrine 4-sulfate
A technology of norepinephrine and a synthetic method, applied in the field of drug synthesis, can solve the problems of low yield, troublesome purification, inability to synthesize a large amount, etc., and achieve the effects of high yield, simple processing and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
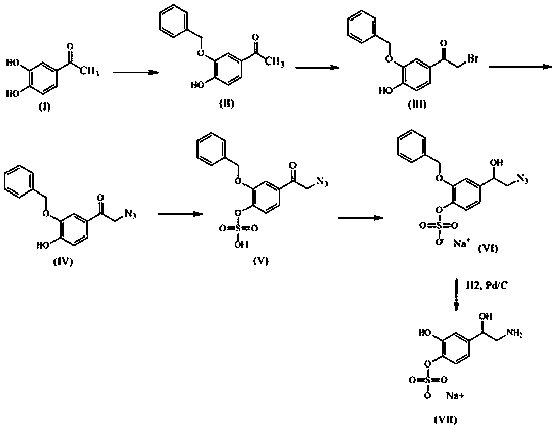
[0018] like figure 1 Shown is the reaction flow diagram of the present invention:
[0019] (1) Suspend 12 g of sodium hydride (60%) in 80 mL of DMSO, stir in an ice bath for 0.5 hours, add 20 g of compound I purchased, stir at room temperature for 5 hours, then add 17 g of benzyl chloride to the reaction solution, After the dropwise addition was completed, react at room temperature for 24 hours; add 200 mL of water to the reaction solution, extract with dichloromethane, concentrate the dichloromethane phase, and purify by column chromatography to obtain 18 g of intermediate II as a white solid.
[0020] (2) Dissolve 16 g of intermediate II in 240 mL of tetrahydrofuran, add 34 g of tetrabutylammonium tribromide at room temperature, and react at room temperature for 24 hours to obtain a red suspension; spin the solvent to dryness, add 200 mL of water, and wash with acetic acid Ethyl extraction was carried out, the ethyl acetate phase was concentrated, and purified by column chr...
Embodiment 2
[0027] like figure 1 Shown is the reaction flow diagram of the present invention:
[0028] (1) Suspend 12 g of sodium hydride (60%) in 80 mL of DMSO, stir in an ice bath for 0.5 hours, add 20 g of compound I purchased, stir at room temperature for 5 hours, then add 16 g of benzyl chloride to the reaction solution, After the dropwise addition was completed, react at room temperature for 24 hours; add 200 mL of water to the reaction solution, extract with dichloromethane, concentrate the dichloromethane phase, and purify by column chromatography to obtain 18 g of intermediate II as a white solid.
[0029] (2) Dissolve 16 g of intermediate II in 240 mL of carbon tetrachloride, add 34 g of N-bromosuccinimide at room temperature, and react at room temperature for 30 hours to obtain a red suspension; spin the solvent dry and add 200 mL of water, extracted with ethyl acetate, concentrated the ethyl acetate phase, and purified by column chromatography to obtain 14 g of intermediate I...
Embodiment 3
[0036] like figure 1 Shown is the reaction flow diagram of the present invention:
[0037] (1) Suspend 12 g of sodium hydroxide (60%) in 80 mL of DMSO, stir in an ice bath for 0.5 hours, add 20 g of compound I, and stir at room temperature for 5 hours, then add 16 g of benzyl bromide to the reaction solution , after the dropwise addition was completed, react at room temperature for 24 hours; add 200 mL of water to the reaction solution, extract with dichloromethane, concentrate the dichloromethane phase, and purify by column chromatography to obtain 18 g of intermediate II, a white solid;
[0038] (2) Dissolve 16 g of intermediate II in 240 mL of acetonitrile, add 34 g of N-bromosuccinimide at room temperature, and react at room temperature for 30 hours to obtain a red suspension; spin the solvent dry, add 200 mL of water, It was extracted with ethyl acetate, the ethyl acetate phase was concentrated, and purified by column chromatography to obtain 14 g of intermediate III as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com