Method and device for co-producing dimethyl carbonate and dimethyl oxalate
A technology of dicarbonic acid and dioxalic acid, applied in the purification/separation of carbonate/haloformate, preparation of carbonate/haloformate, chemical instruments and methods, etc., can solve the problem of high methanol content, carbonylation The problems of unfavorable reaction and high energy consumption of separation can achieve the effect of saving equipment investment costs, reducing energy requirements and operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
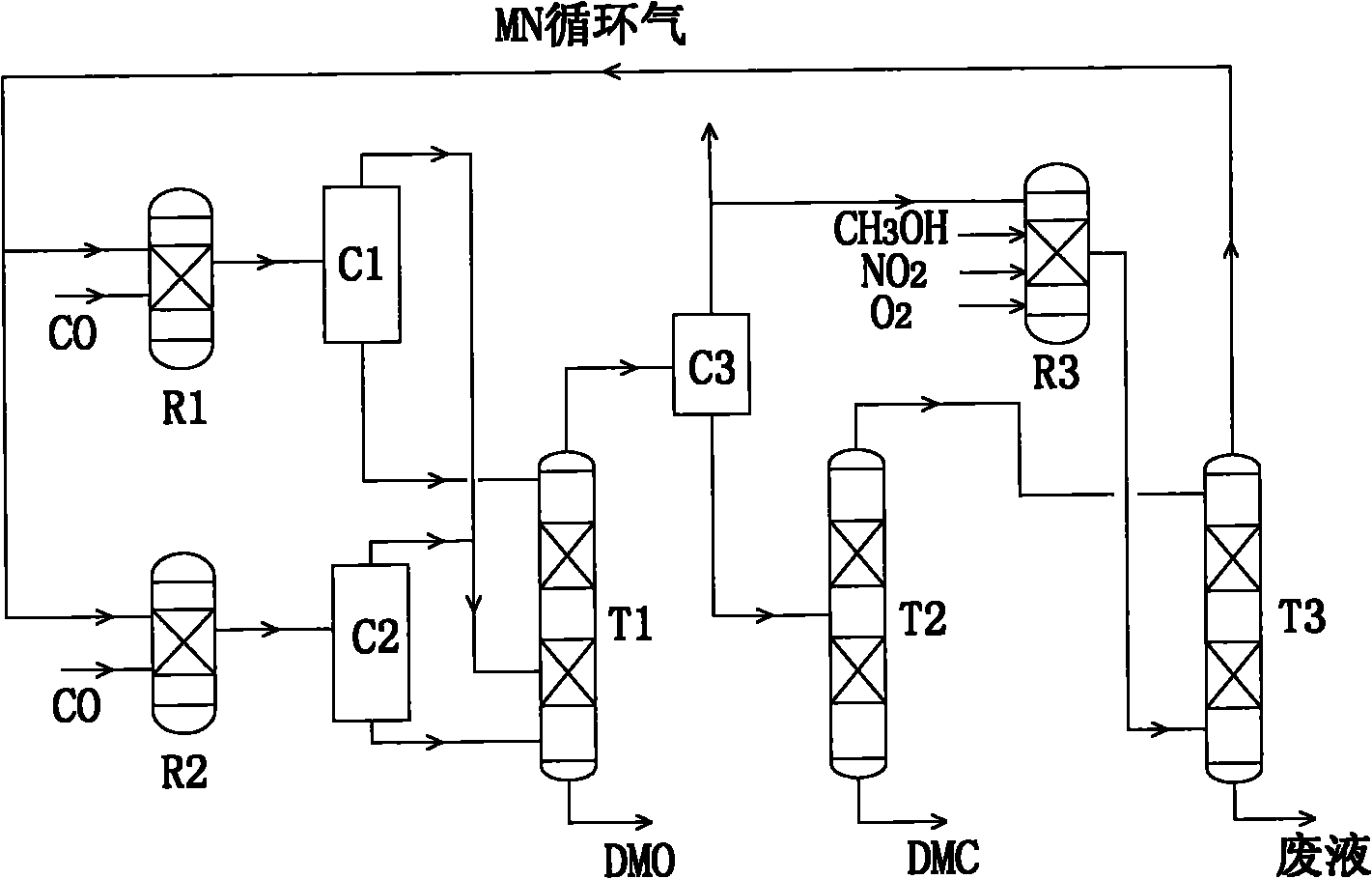
[0052] Purify raw gas CO 18.01Kmol / hr (99%) and cycle gas 244.73Kmol / hr (containing methanol 1.4mol%, DMC 0.4mol%, NO 1.4mol%, MN 16.2mol%, CO 5.8mol%, N 2 74.8mol%) according to the volume ratio of 0.6556: 1 is divided into two stocks and mixed respectively, then enters the DMC reactor (90 ℃, 1.2atm, Pd / Al 2 o 3 catalyst) and DMO reactor (120°C, 1.2atm, Pd / Al 2 o 3 Catalyst) reaction, the respective products are condensed and separated by C1 (30°C) and C2 (60°C) respectively, and the gas parts are mixed and sent to the lower part of the DMO rectification column (operating pressure 1.5atm, reflux ratio 0.8, theoretical plate number 10) On about the 8th tray, the liquid part from C1 is sent to the top of T1, and the liquid part from C2 is sent to about the 9th tray at the bottom of T1. After rectification, the DMO product can be obtained at the bottom of the T1 tower (yield 5.868 Kmol / hr, purity 99.5%, temperature 181.8 DEG C. Containing 1.4% methyl alcohol and 2.0% DMC (9.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com