Detection method of ceftiofur and application thereof
A detection method, the technology of ceftiofur, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of unsuitable rapid detection of ceftiofur, many processing steps, and high toxicity, and achieve low toxicity, simple pretreatment, The effect of using less reagent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
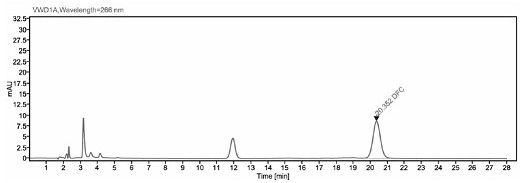
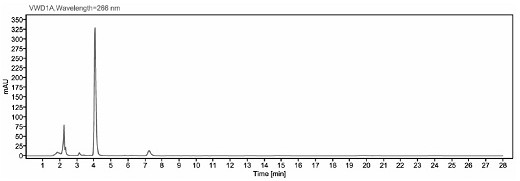
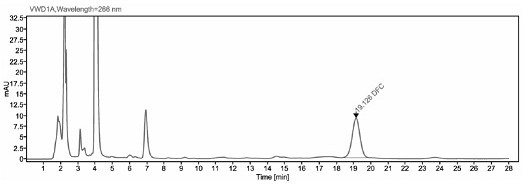
[0041] The detection method of ceftiofur in pig plasma in embodiment 1
[0042] 1. Preparation of the main solution
[0043] 0.05 mol / L boric acid buffer solution: Weigh 19 g boric acid and 3.7 g potassium chloride, add water to dissolve and dilute to 1000ml.
[0044] Extract (0.4% DTE w / v): Weigh 1.0 g of dithioerythritol (DTE), dissolve it in 250 ml of borate buffer, and prepare immediately after use.
[0045] 0.1% trifluoroacetic acid-water solution: Take 1 ml trifluoroacetic acid and add it to 1000 ml ultrapure water, mix well.
[0046] 1 mg / ml DFC standard stock solution: Weigh about 10 mg of DFC standard into a 10 ml brown volumetric flask, dissolve with methanol and dilute to volume, store at -70°C for later use.
[0047] 2. Sample pretreatment
[0048] Take 200 μl of plasma sample, add 400 μl of extract solution (0.4% DTE w / v), vortex mix, and 50°C water bath for 30 min, and vortex mix once every 10 min. After the water bath was completed, the sample was centrifuge...
Embodiment 2
[0088] Embodiment 2 Pharmacokinetic comparative test of three kinds of ceftiofur injections in pigs
[0089] There were 18 healthy Landrace binary hybrid pigs, weighing 90-110 kg. Before the experiment, the rats were fed for 1 week as usual, and the feed was a diet without any antibiotics. Before the experiment, the pigs used in the experiment were screened, and the age, weight, and fat condition were as consistent as possible. Using a single-center, completely randomized, single-period parallel, single-administration experimental design, 18 healthy Landrace binary crossbreed pigs were divided into the first, second, and third groups, a total of three groups, with 6 pigs in each group, male and female. Half.
[0090] Fasted for 12 h before administration, but provided adequate drinking water. The next morning on an empty stomach, the pigs in the first, second and third groups were injected intramuscularly at a dose of 5 mg / kg b.w. (calculated as ceftiofur), and the first gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com