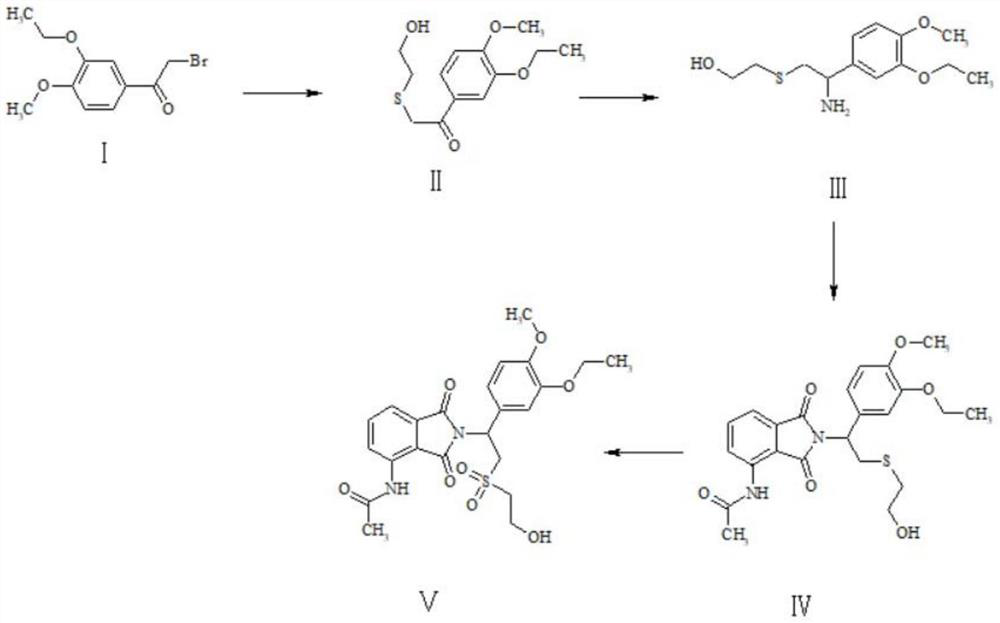
A kind of preparation method of Apremilast impurity
A technology of impurities and products, applied in the field of preparation of Apremilast impurities, to achieve the effect of good environmental protection, high product purity and simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
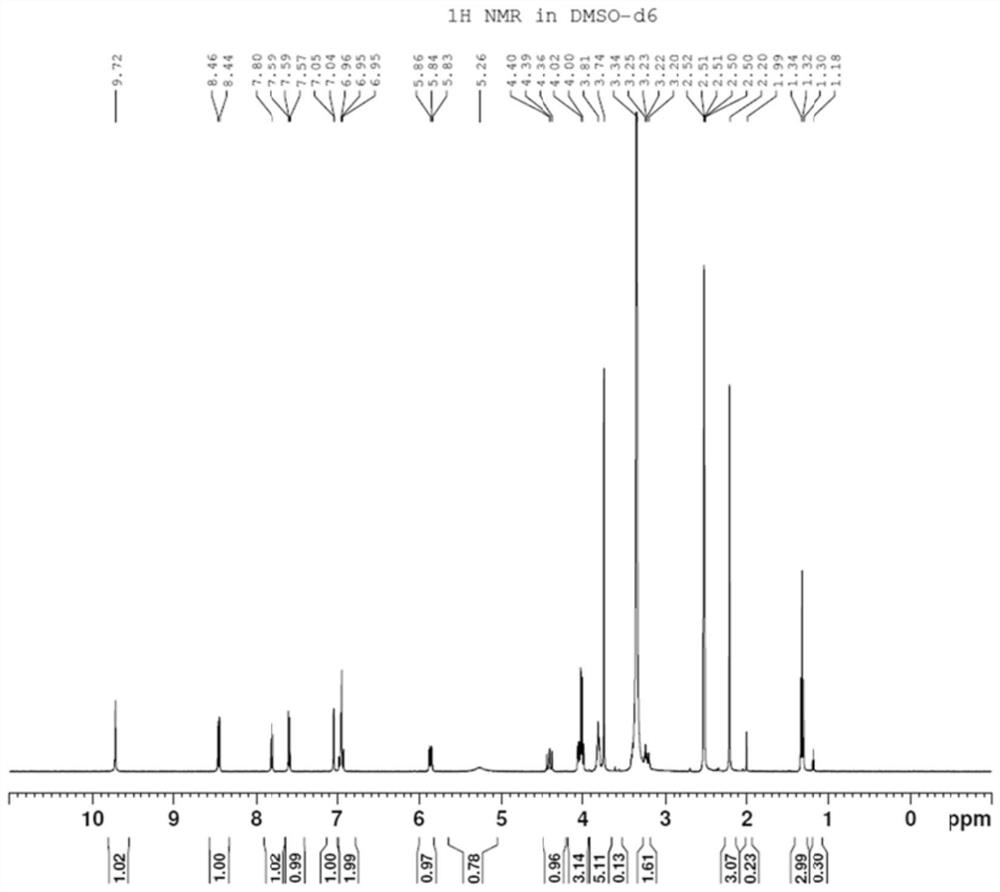
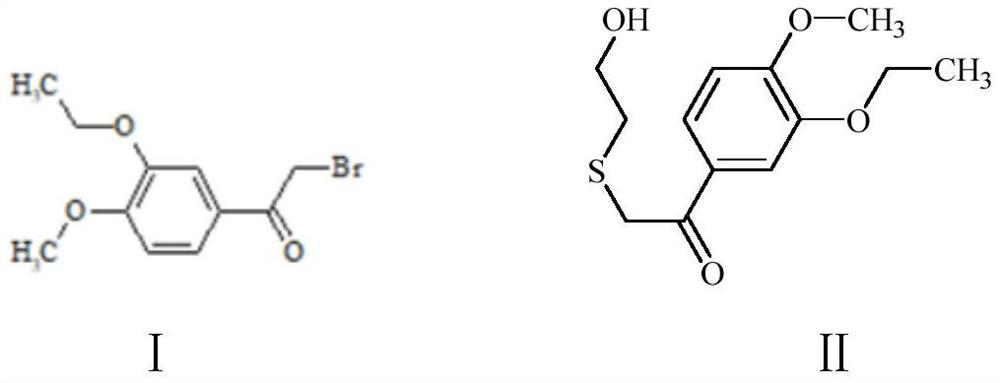
[0031] Preparation of Compound II: Dissolve 25g of Compound I in 200mL of N,N-dimethylformamide, add 21.4g of 2-mercaptoethanol, then add 15g of sodium hydroxide, and react at 100°C for 12 hours. After the reaction is complete, the reaction solution is filtered. The filtrate was spin-dried, and the crude product was purified through a column with dichloromethane and methanol to obtain 21.5 g of yellow solid II with a yield of 87%.
[0032]
[0033] Preparation of compound III: Dissolve 21 g of compound II in 200 mL of tetrahydrofuran, add 12 g of ammonium formate and 4 g of sodium triacetoxyborohydride at room temperature, react at room temperature for 8 hours, monitor the end of the reaction by TLC, spin the reaction solution to dryness, and add 200 mL of water , add 1mol / L sodium hydroxide under ice bath to adjust pH=12, extract three times with dichloromethane, 50mL each time, combine the organic layers and dry with anhydrous sodium sulfate, filter, spin the filtrate to o...
Embodiment 2
[0040] Preparation of compound II: Dissolve 23g of intermediate I in 200mL N,N-dimethylformamide, add 20g of 2-mercaptoethanol, then add 18g of potassium hydroxide, react at 100°C for 12 hours, the reaction is completed, the reaction solution is filtered, and the filtrate The crude product was spin-dried to obtain a crude product, which was purified through a column with dichloromethane and methanol to obtain 18.6 g of yellow solid II with a yield of 81.7%.
[0041]
[0042] Preparation of Compound III: Dissolve 17g of Compound II in 150mL of tetrahydrofuran, add 19g of ammonium formate at room temperature, react for a period of time at room temperature, then add sodium borohydride for reduction, TLC monitors the end of the reaction, spin the reaction solution to dryness, add 200mL of water, Add 1mol / L sodium hydroxide under ice bath to adjust the pH=12, extract three times with dichloromethane, combine the organic layers and dry with anhydrous sodium sulfate, filter, spin t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com