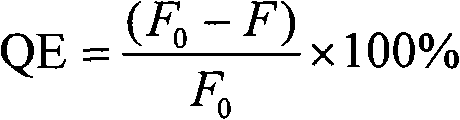Patents
Literature
36results about How to "Simple sample handling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for simultaneously detecting forty types of amino acids in dried blood spots, blood and urine
InactiveCN108333268AAvoid distortionRealize real-time monitoringComponent separationThree levelChromatographic separation
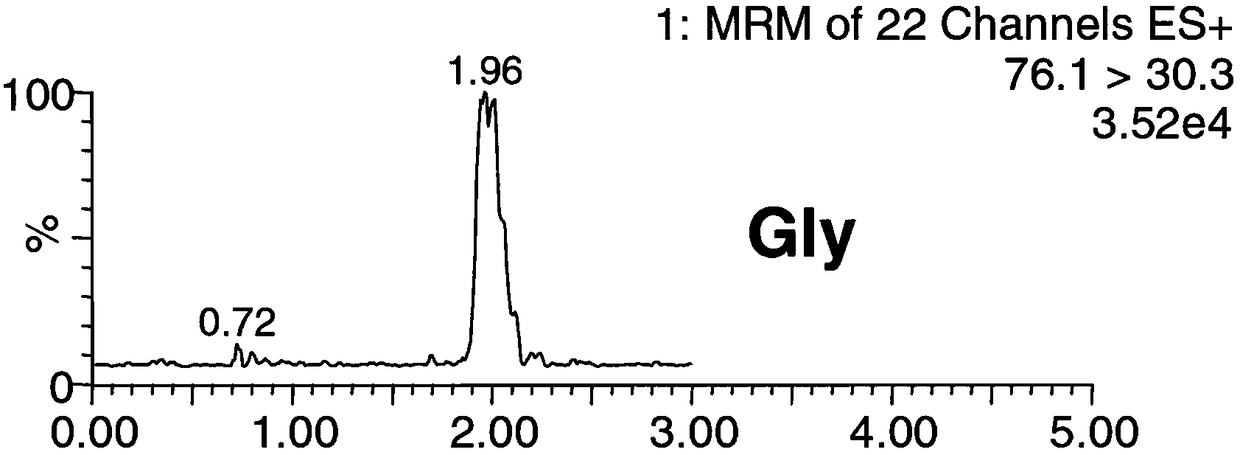
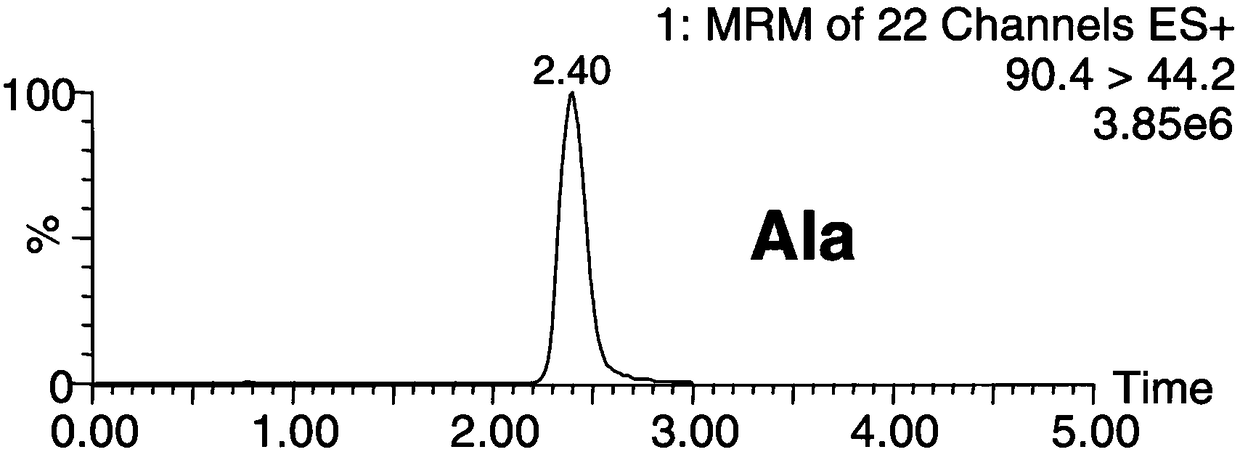
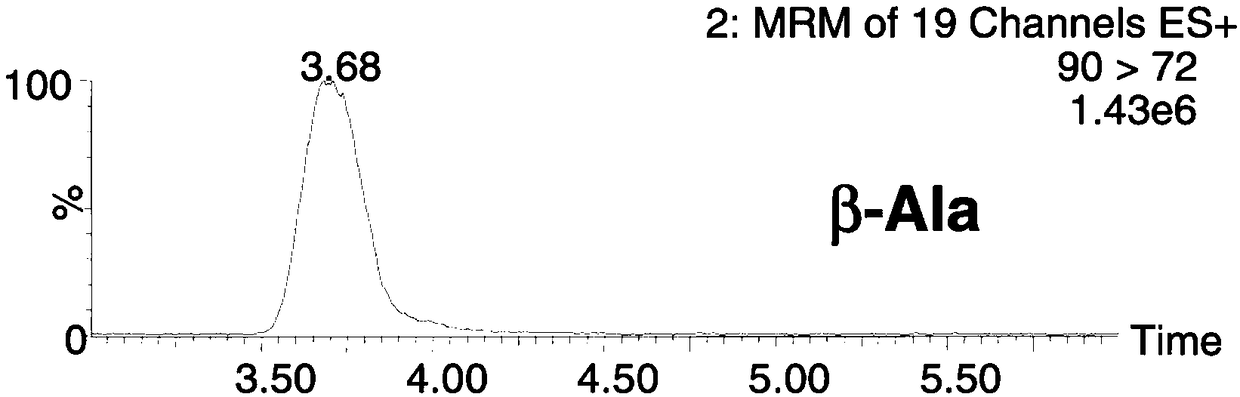
The invention discloses a method for simultaneously detecting forty types of amino acids in dried blood spots, blood and urine. The method comprises the following steps of firstly, pretreating a biological sample by a simple liquid and liquid extracting method; performing chromatographic separation and mass spectrometric detection; using the relative retention time and qualitative ions as the qualitative basis for each type of amino acid, and using a standard product to manufacture a standard curve for quantifying. The method has the advantages that the accuracy and validity of the method shall be studied by three levels of quality control products, so as to avoid the distortion of detection results; the forty types of non-derived amino acids in one sample can be simultaneously detected bya HPLC-MS / MS (high performance liquid chromatography-mass spectrograph) technique for the first time; the operation is simple, convenient and rapid, the flux is high, and the cost is low; the level of amino acids in a human body can be effectively monitored in real time; the enough basis is provided for the clinical disease diagnosis; the method is suitable for being clinically popularized and applied.
Owner:JINAN YING SHENG BIOTECH
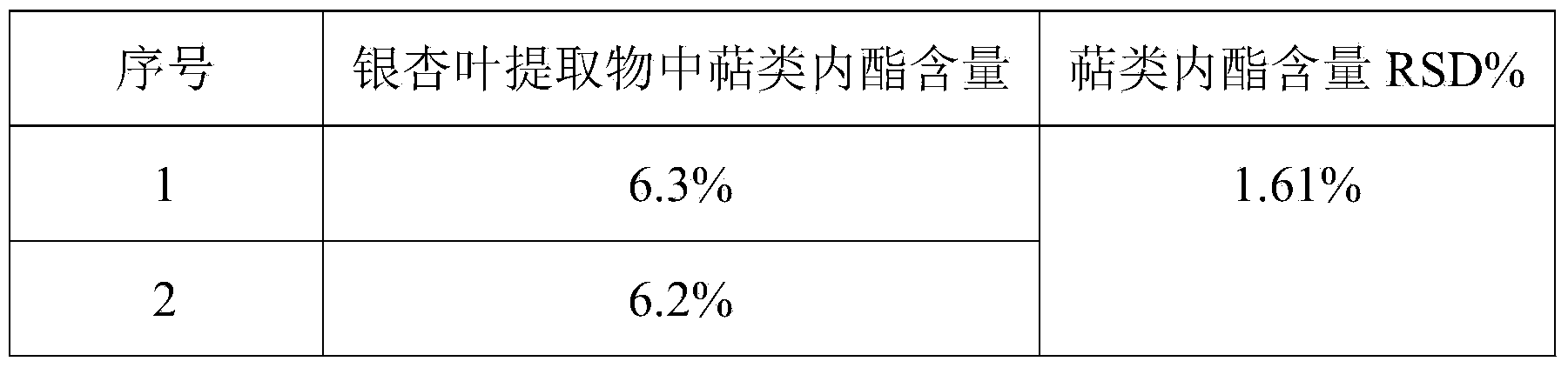
Method for detecting terpene lactones in ginkgo biloba extract
ActiveCN104034826ASimple sample handlingImprove detection efficiencyComponent separationTerpene lactonesChemistry
The invention discloses a method for detecting terpene lactones in a ginkgo biloba extract. The ginkgo biloba extract is the raw material and is stirred and extracted through ethyl acetate in a magnetic mode, and a solution is obtained; rotation steaming and concentration are conducted on the solution until the solution is dried, methyl alcohol is used for dissolution, ultrasonic treatment is conducted, the solution serves as a detection sample, and a high performance liquid chromatography-evaporative light-scattering detector is use for analyzing and detecting. According to the method, a detection result is consistent with a detection result in a Chinese pharmacopoeia method, in extracting and separating of the terpene lactones of the ginkgo biloba, the method is more convenient to use and practical. The time for detecting each sample is lower than 12 min (time consumed in the Chinese pharmacopoeia method is 45 min), the detection efficiency is greatly improved, a test cycle is shortened, cost is saved, and the method is economical and environmentally friendly. The method can be used for qualitative and quantitative analysis of the lactones of the ginkgo biloba. It is verified and shown by the methodology that the method is good in separating effect, and the requirements for method linearity, stability, repeatability, the sample adding recycling rate and the like are met. The method is further suitable for detecting preparations with one or more kinds of ginkgo biloba terpene lactones as the raw material, suitable for industrial production and application and large in application value.
Owner:SHANGHAI SINE PROMOD PHARMA
Clinically intelligent diagnostic devices and methods
InactiveUS20060240453A1Reduce testing costsRapid diagnosisBioreactor/fermenter combinationsMaterial nanotechnologyIntelligent designAnalyte
The invention relates to the clinically intelligent design of diagnostic devices (such as microarrays) and methods of making and using such devices in differential diagnoses of specific clinical symptoms or sets of symptoms. In one aspect, the devices include various probes used to perform parallel screening of a number of analytes. The probes are clustered on the devices based on known clinical presentations of symptoms associated with specific diseases and disorders.
Owner:INTELLIGENT MEDICAL DEVICES
Method for determination of fat-soluble vitamins by using semi-automatic sample treatment liquid chromatographic technology
ActiveCN106404947ASimple sample handlingHigh throughputComponent separationChemistryFat-Soluble Vitamin
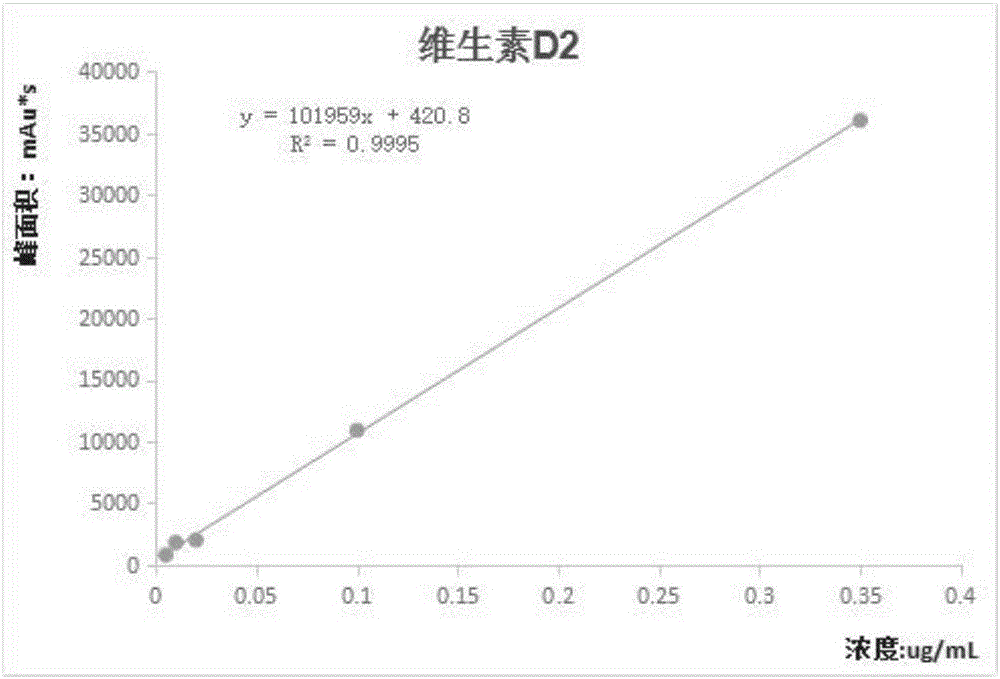
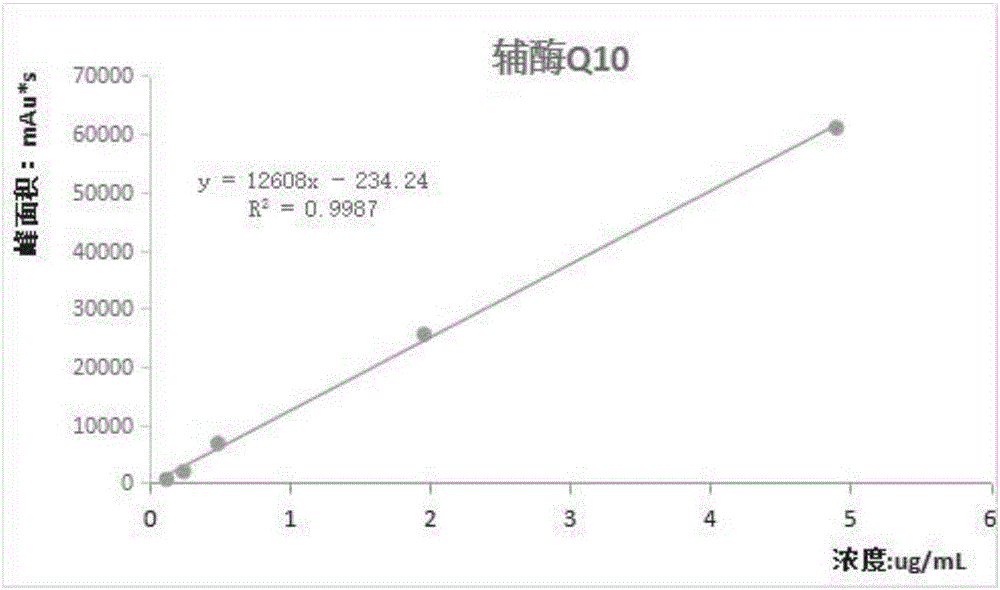
The invention discloses a method for simultaneous determination of various fat-soluble vitamins in serum by using a semi-automatic sample treatment liquid chromatographic technology; the HPLC method is adopted, and the chromatographic conditions comprise that a mobile phase A is a 0.05% trifluoroacetic acid aqueous solution, a mobile phase B is a 0.02% trifluoroacetic acid methanol-ethanol mixed solution, and gradient elution is adopted; the various fat-soluble vitamins are selected from vitamin A, vitamin D2, vitamin D3, alpha-tocopherol, beta-tocopherol, gamma-tocopherol, delta-tocopherol, K1, K2 or Q10. Through optimization of a sample pretreatment method and high performance liquid chromatographic conditions, the method for simultaneous determination of the various fat-soluble vitamins in the serum is established, can carry out precise qualitative and quantitative analysis, is a detection method having the advantages of simple and fast sample processing, high flux, reliable result and low cost, has the advantages of strong specificity, high sensitivity, simple operation, low cost and the like, has easily analyzed and objective results, and is especially suitable for clinical popularization and application.
Owner:济南国益生物科技有限公司
A rapid identification method for judging whether Radix Paeoniae Alba has been fumigated with sulfur
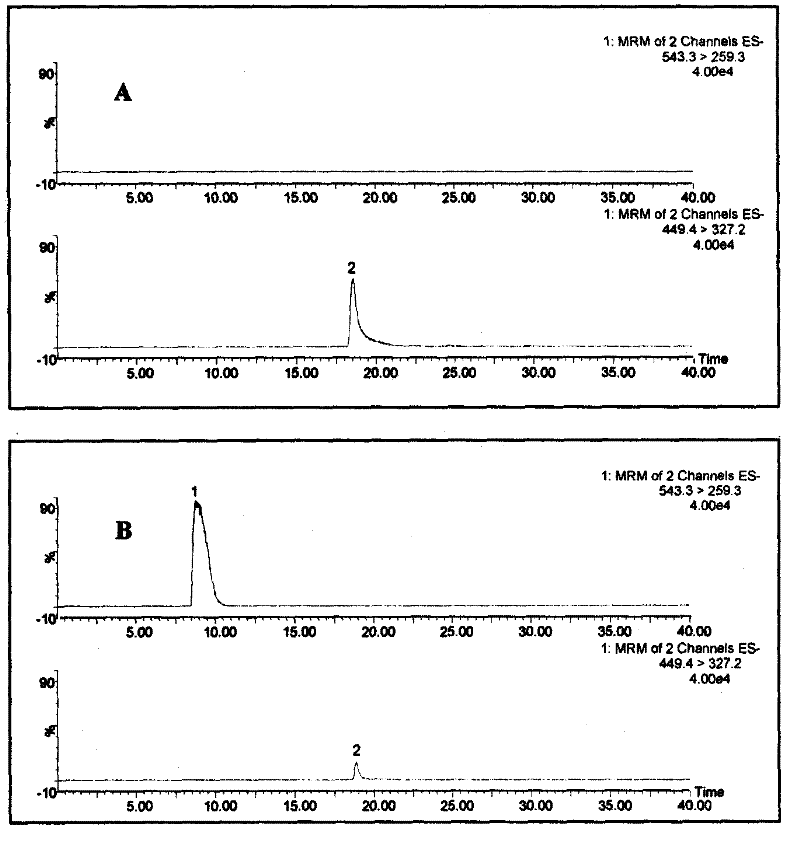
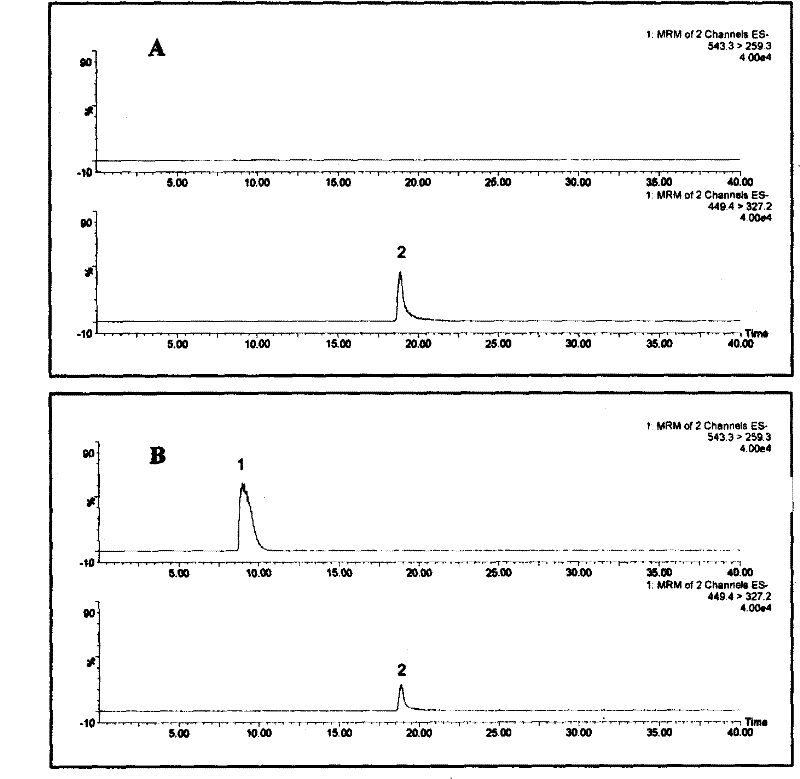
InactiveCN102288713ASmall amount of samplingSimple sample handlingComponent separationIonMass spectrum analysis
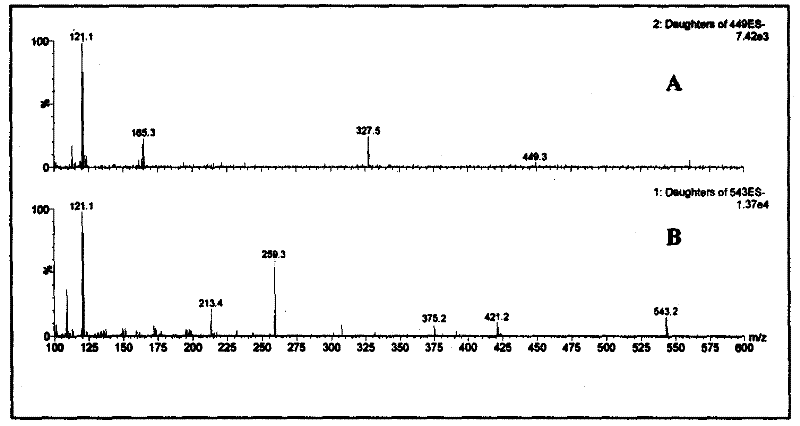
The invention belongs to the field of authenticity identification of Chinese medicinal materials and decoction pieces, in particular to a method for identifying the quality of Chinese medicinal materials and decoction pieces by using a specific analysis method to detect characteristic chemical components. Illegal acts of sulfur fumigation of Radix Paeoniae Alba occur from time to time, and a rapid identification method is urgently needed. The present invention is based on the conversion of paeoniflorin into paeoniflorin sulfide after sulfur fumigation of Radix Paeoniae Alba, using 50% methanol to ultrasonically extract Radix Paeoniae Alba samples, using reversed-phase liquid chromatography-triple quadrupole mass spectrometry analysis technology for determination, by observing the Radix Paeoniae Alba Whether there are two groups of multi-reaction ion m / z449.4→m / z327.2 and m / z543.3→m / z259.3 chromatographic peaks in sequence in the chromatogram of Paeoniae Alba samples can be used to judge whether Paeoniae Alba is fumigated with sulfur. If there are chromatographic peaks whose reaction ions are m / z543.3→m / z259.3 and m / z449.4→m / z327.2 in the following order, it means that the Radix Paeoniae Alba sample has been fumigated with sulfur. If the chromatographic peak of m / z449.4→m / z327.2 does not appear, and the chromatographic peak of m / z543.3→m / z259.3 does not appear, it can be judged that the white peony root has not been fumigated with sulfur. The invention has the outstanding advantages of small sampling amount (can be as low as 2 mg), high sensitivity, strong specificity, and no need to use paeoniflorin sulfide which is not easily obtained as a reference substance.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Test strip for measuring protein content of food and testing method thereof
InactiveCN101975862ASimple sample handlingThe detection process is fastScattering properties measurementsBiological testingPhysical chemistryColor measurement
The invention provides a test strip for measuring protein content of a food and a testing method thereof. The test strip comprises a filter strip, a reaction strip, a water-absorbing strip and a substrate, wherein an indicator is adsorbed in the reaction strip and can perform specific reaction with protein in a sample; and the protein content is indicated by color changing. By using the test strip, the protein is tested by a portable color measurement instrument, the testing time is short and is generally less than 30 seconds, the result is accurate and rapid field testing of the protein in the food can be realized.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Device for staining 3D biopsy tissue
PendingUS20210270705A1Simple sample handlingReduce frictionSurgical needlesPreparing sample for investigationStainingTissue sample
A method and system for processing a 3D tissue sample is provided, comprising the steps of receiving a tube with an inner space and two open ends, wherein the tube is configured to retain the 3D tissue sample in the inner space, arranging the tube so that one of the two open ends of the tube is located at a fluid channel, and forcing or actively pressing a tissue processing agent through the fluid channel and into the tube so that the tissue processing agent passes through the tissue.
Owner:KONINKLJIJKE PHILIPS NV
Method for identifying cigarettes by using electrochemical fingerprints
InactiveCN103048365ASimple sample handlingReduce testing costsMaterial electrochemical variablesStandard samplesArtificial neuronal network
The invention relates to a method for identifying cigarettes by using electrochemical fingerprints. The method comprises the steps that cut tobacco of cigarettes of different varieties and cigarettes to be identified are taken respectively, dried and cooled, and then are stirred by adding an extracting solution inside and filtered, and the filtrate is collected, or the filtered cut tobacco is cooled and then ground into powders to obtain a standard sample and a sample to be identified; then an appropriate amount of the standard sample or the sample to be identified is taken and added into a B-Z oscillating system with a constant temperature, and the electrochemical fingerprints are obtained with a platinum electrode as the indicating electrode and a double salt bridge saturated calomel electrode or Ag / AgCl electrode as the reference electrode; and the characteristic parameters of the standard sample and the sample to be identified are extracted according to the electrochemical fingerprints, and the category or the genuine or whether the counterfeit of the sample to be identified is a counterfeit is identified by using such as pattern recognition methods as an artificial neural network. With the method of the invention, the treatment of samples is simple, the cost is low, the identification speed is fast, the operation is simple and convenient, and the results are accurate and reliable; and the method can be used for identifying the varieties and whether the cigarette is a counterfeit.
Owner:SOUTHWEST UNIVERSITY
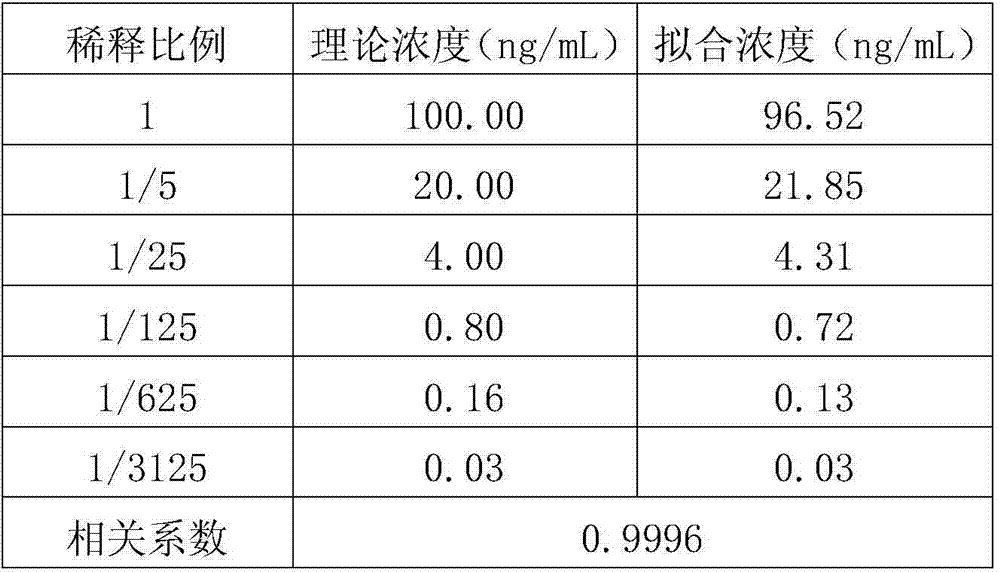
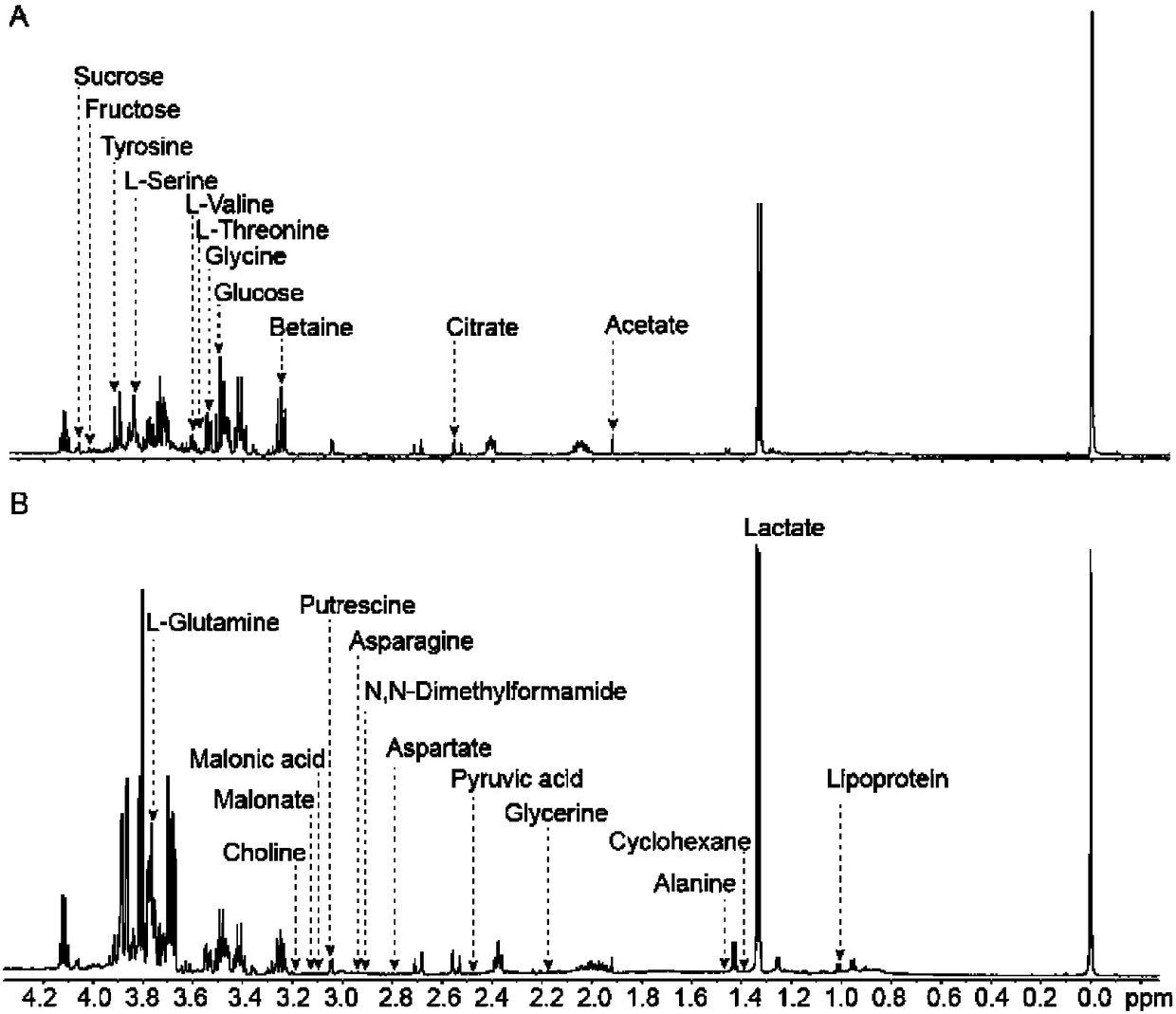
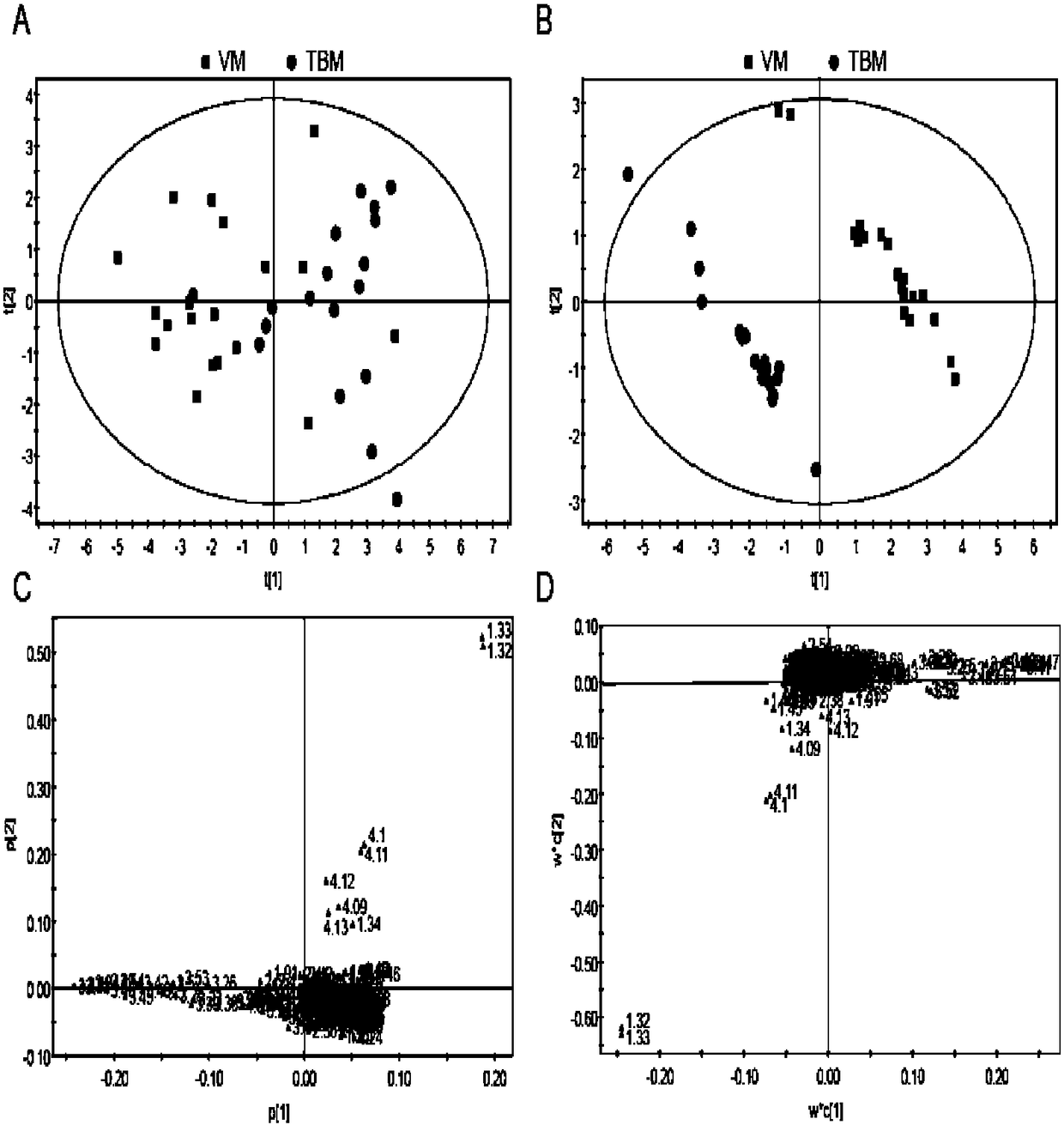
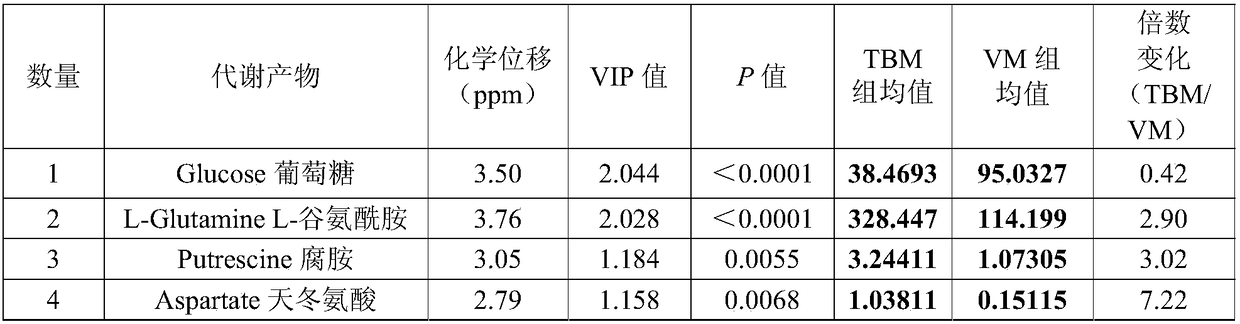
Method for distinguishing tubercular meningitis and virus meningitis based on nuclear magnetic resonance technology and application thereof
ActiveCN105943048AEasy to handleNon-destructiveDiagnostic recording/measuringSensorsNMR - Nuclear magnetic resonanceMetabolite
The invention discloses a method for distinguishing tubercular meningitis and virus meningitis based on a nuclear magnetic resonance technology and application thereof. According to the method, nuclear magnetic resonance detection is conducted on the cerebrospinal fluid of a patient to be detected to obtain relative expression quantities of difference metabolites represented by chemical shifts of 3.50 ppm, 3.76 ppm, 3.05 ppm, and 2.79 ppm respectively, and then whether the patient suffers from the tubercular meningitis or the virus meningitis is distinguished according to the relative expression quantities of the difference metabolites. Compared with the prior art, the method has the advantages of being simple in sample treatment, free of sample damage, small in sample quantity, good in repeatability, low in cost and the like.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV +1
Method for distinguishing fungi and bacteria
ActiveCN101831054AFast processingSimple sample handlingMicrobiological testing/measurementFluorescence/phosphorescenceFörster resonance energy transferVisibility
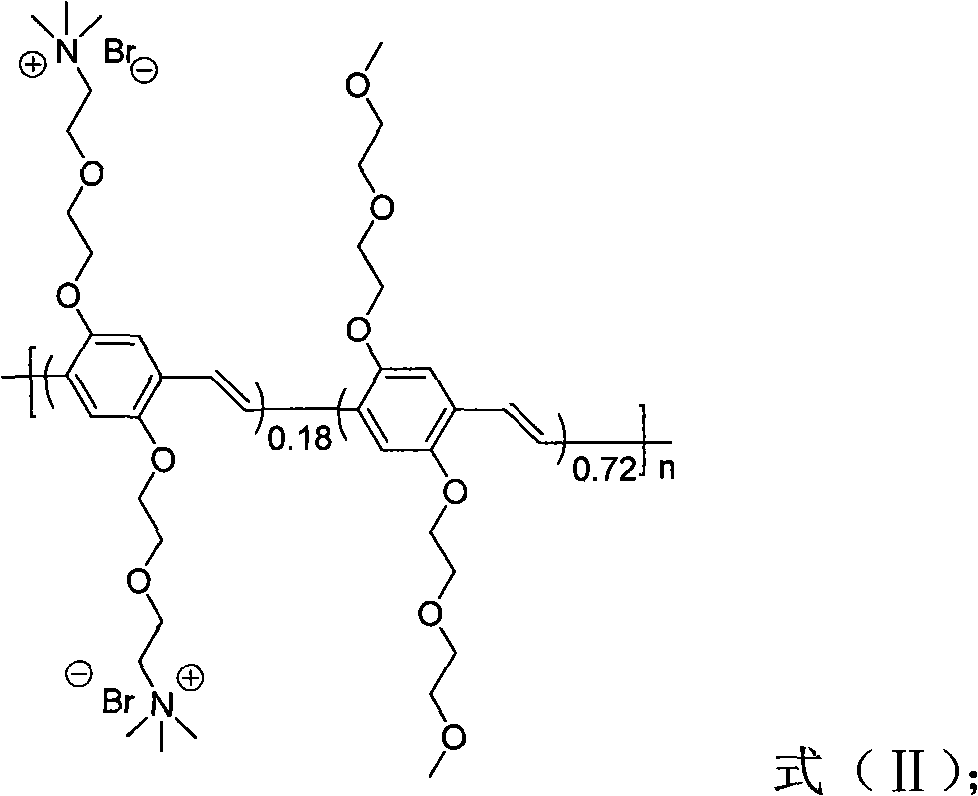
The invention discloses a method for distinguishing fungi and bacteria, which comprises the following steps of: detecting whether the bacteria to be detected can induce the compounds expressed by the formulas (I and II) to cause fluorescence resonance energy transfer; if the bacteria to be detected can induce the compounds expressed by the formulas (I and II) to cause fluorescence resonance energy transfer, determining that the bacteria to be detected is bacteria; and if the bacteria to be detected cannot induce the compounds expressed by the formulas (I and II) to cause fluorescence resonance energy transfer, determining that the bacteria to be detected is fungi. The method has the advantages of high speed, visibility and convenience and the like. The method can be used for distinguishing the types of the infectious pathogenic bacteria, instructing the administration of the doctors aiming at different patients, improving the therapeutic efficiency and reducing the occurrence of drug resistance caused by incorrect use of antibiotic. The method also has application value in aspects of food safety, environmental monitoring and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI
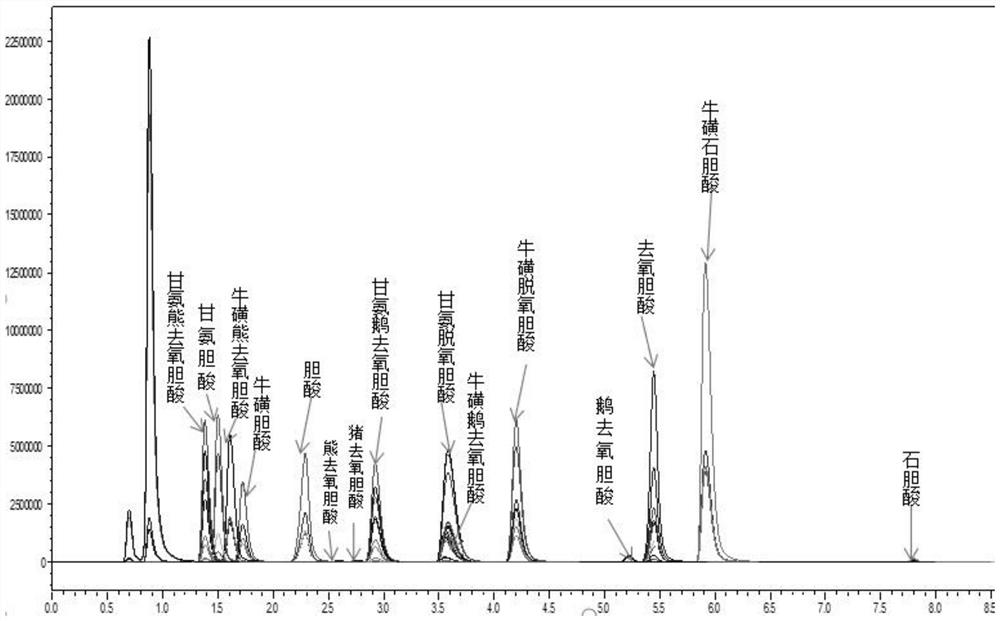
Method and kit for simultaneously detecting various bile acids in blood sample
PendingCN113009061AImprove throughputReliable resultsComponent separationChromatographic separationSerum samples
The invention discloses a method and a kit for simultaneously detecting various bile acids in a blood sample, and belongs to the technical field of bile acid detection. The invention discloses a method for simultaneously detecting various bile acids in a blood sample, which comprises the following steps: pretreating the sample, performing chromatographic separation and mass spectrometric detection, respectively selecting a pair of qualitative ions and a pair of quantitative ions for each bile acid, and determining the content of various bile acids in the blood sample by taking the relative retention time of each bile acid and the qualitative ion pair as a qualitative basis; and making a standard curve by using a standard product for quantification. Meanwhile, the method provided by the invention is accurate and effective by applying three levels of quality control product inspection methods, and detection result distortion is avoided. According to the method, the aim of simultaneously detecting fifteen bile acids in a serum sample by applying the LC-MS technology is achieved for the first time, the influence of interferents is reduced, the method is simple, convenient and rapid to operate, the bile acid level in a human body is effectively monitored, and the method has guiding significance on reasonable and safe supplement of the bile acids and is easy to clinically popularize and popularize.
Owner:广东南芯医疗科技有限公司 +1
Method for determinating seven kinds of quinolone residues in animal-source food through membrane dialysis-high performance liquid chromatography and tandem mass spectrometry
InactiveCN104316615ASimple sample handlingHigh sensitivityComponent separationTandem mass spectrometryMicrogram
The invention belongs to the field of chemical analysis, and relates to a method for determinating seven kinds of quinolone residues in animal-source food through membrane dialysis-high performance liquid chromatography and tandem mass spectrometry. Through a membrane dialysis technology, a high performance liquid chromatography and tandem mass spectrometry (HPLC-MS / MS) determination method for determinating the seven kinds of quinolone residues in the animal-source food at the same time is established. A standard regenerated cellulose membrane (RC, molecular weight cut off (MWCO): 1000D) as a dialysis membrane, drug residues penetrate into dialysate through dialysis, and the dialysate is extracted by using chloroform; by the method, all quinolone drugs have good linearity within the concentration range of 5.0-100.0 micrograms / kg and have the limit of quantitation of 5.0 micrograms / kg; at three concentration adding levels of 5.0, 10.0 and 25.0 micrograms / kg, the average recycling ratio is 65.5-84.1%; the variable coefficient is 4.3-11.6%. The method is high in sensitivity, good in reproducibility and economical, and can be applied to determination and confirmation of the seven kinds of the quinolone residues in the animal-source food.
Owner:威海出入境检验检疫局检验检疫技术中心 +1
Quantitative determination method for human serum A beta (amyloid peptide)
ActiveCN103852579AHigh sensitivitySimple sample handlingMaterial analysis by observing effect on chemical indicatorDisease diagnosisPolyclonal antibodiesMouse monoclonal antibody
The invention discloses a quantitative determination method for human serum A beta (Amyloid peptide). The quantitative determination method sequentially comprises the following steps: a) by adopting a kit, preconfiguring a specific rat monoclonal antibody with paved amyloid peptide structure N-terminal amino acid sequence to have capturing immune reaction with amyloid peptide in a plasma sample which is subjected to temperature control and constant-speed centrifugation treatment, to obtain a reaction compound; b) carrying out secondary antigen-antibody reaction to the reaction compound with anti-amyloid peptide specific amino acid sequence rabbit-anti-human amyloid peptide antibody, to obtain a reaction conjugate; c) carrying out color development reaction on the reaction conjugate through a rabbit-anti-rat polyclonal antibody with an enzyme primer, then carrying out quantitative determination through a spectrophotometer. According to the method, the amyloid peptide detection sensitivity can be improved, the sample treatment and operation procedures can be simplified, and the method can provide the laboratory diagnosis for hospital clinical alzheimer disease, disease screening to the crowd carrying dementia genetic history or dementia danger gene, and suspected occult patients, observation / judgment of curative effect of clinical new drugs, and experimental study of universities and colleges.
Owner:姚钧
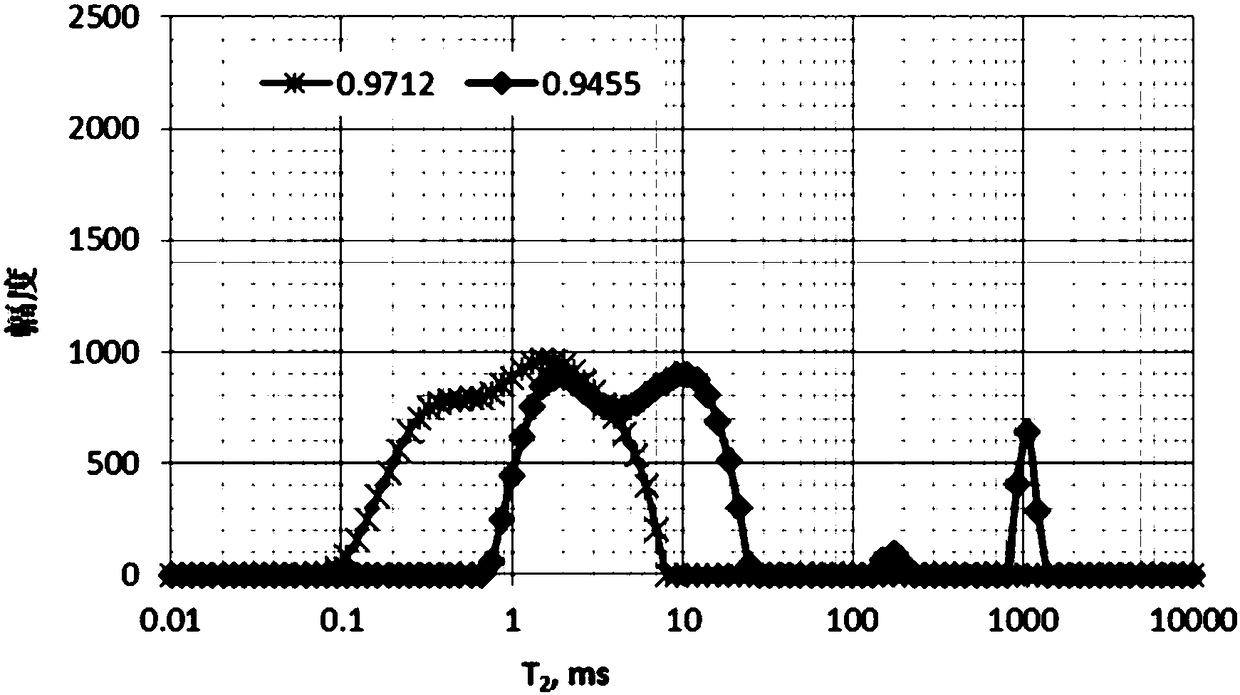
Method for measuring crude oil density
ActiveCN109387456ASimple sample handlingComprehensive considerationSpecific gravity measurementNMR - Nuclear magnetic resonanceSimple sample
The invention discloses a method for measuring crude oil density. The method comprises: 1, placing crude oil to be tested in a preset container and performing constant temperature treatment, 2, carrying out T1-T2 two-dimensional nuclear magnetic resonance analysis on the crude oil subjected to constant temperature treatment to obtain oil peak centroids (T2, T1) of the crude oil to be tested, 3, calculating a ratio of horizontal to vertical coordinates of the oil peak centroids (T2, T1) of the crude oil to be tested to obtain an oil peak centroid coordinate ratio, and based on a relationship curve of a preset oil peak centroid coordinate ratio and density of the crude oil, according to the oil peak centroid coordinate ratio, determining the density of the crude oil to be tested. The methodsolves the problem that the existing method only utilizes the T1 spectrum or the T2 spectrum and has limitation and problems in sample processing complexity, time taking difficulty and narrow application range, has a simple sample processing process in implementation, considers more comprehensive factors and is free of sample degassing, sample weighing or volume measurement.
Owner:CHINA PETROLEUM & CHEM CORP +1
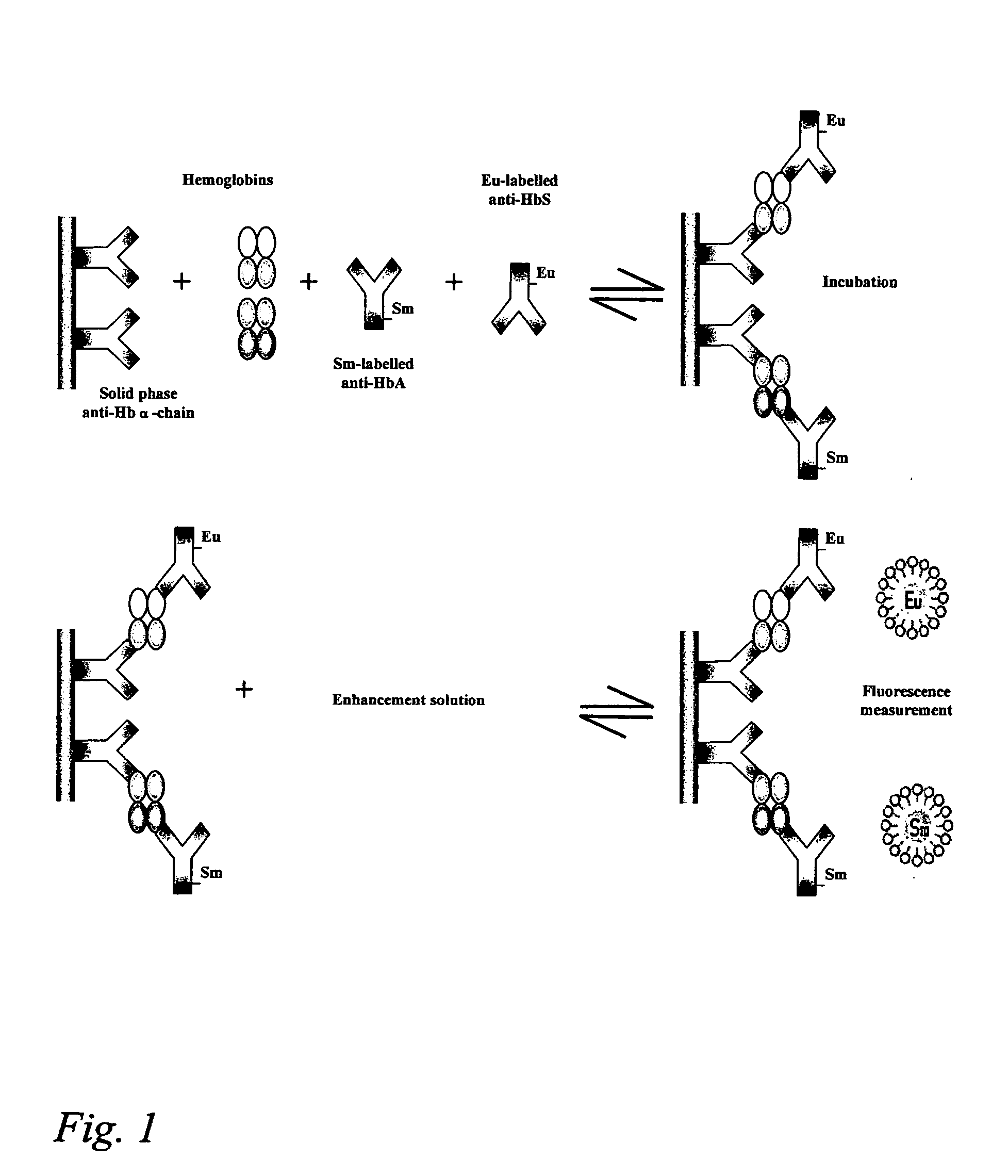
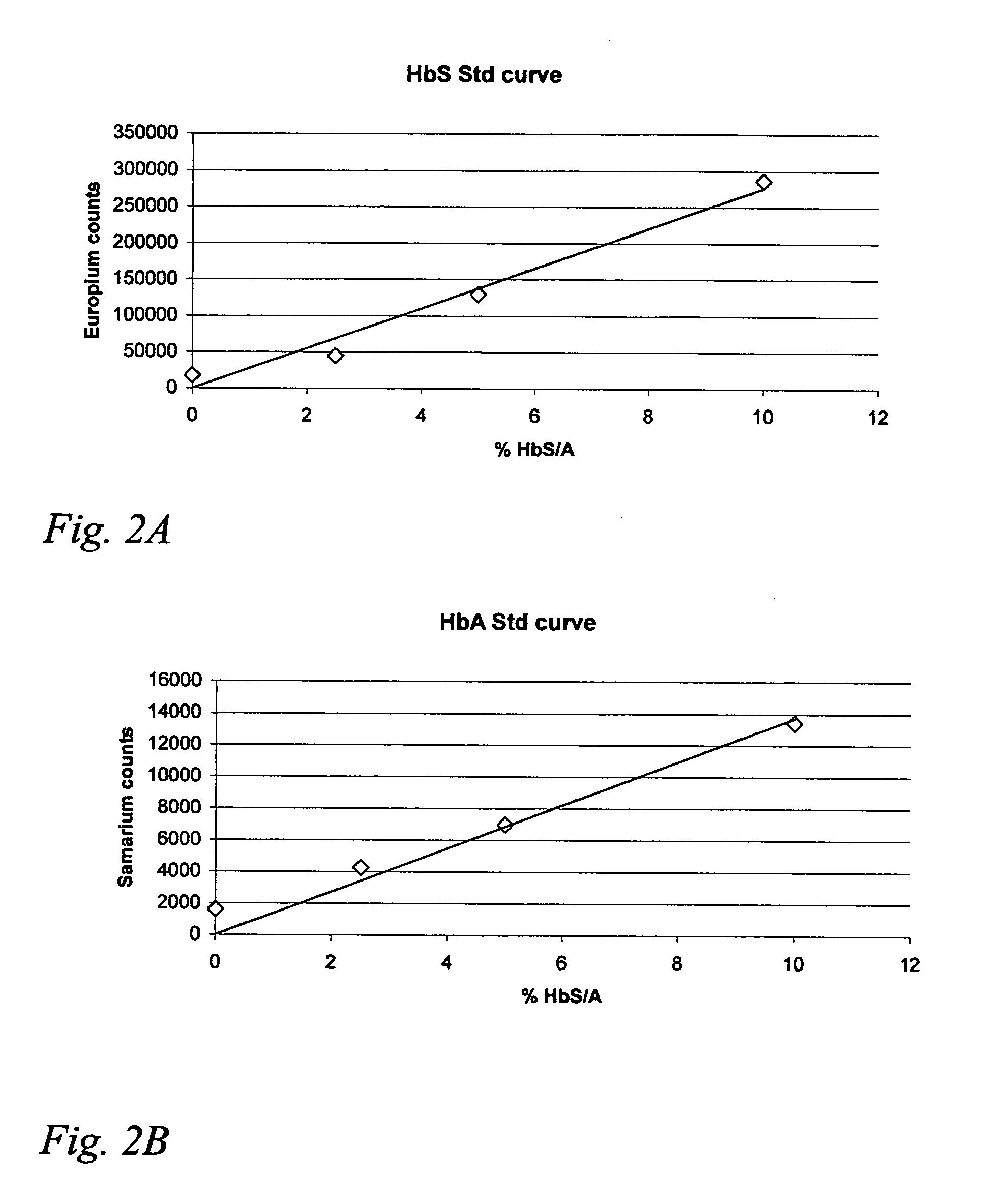
Hemoglobin assay for neonatal screening
InactiveUS20050118654A1Simple methodAvoid pollutionHybrid immunoglobulinsChemiluminescene/bioluminescenceHemoglobinopathyPediatrics
The present invention provides an assay, suitable for neonatal screening of hemoglobinopathies by quantitatively determining the presence of at least one pair of hemoglobin (Hb) variants, determining the ratio between the signals obtained from each variant; and, based on that ratio, determining whether the tested subject is afflicted or not.
Owner:WALLAC
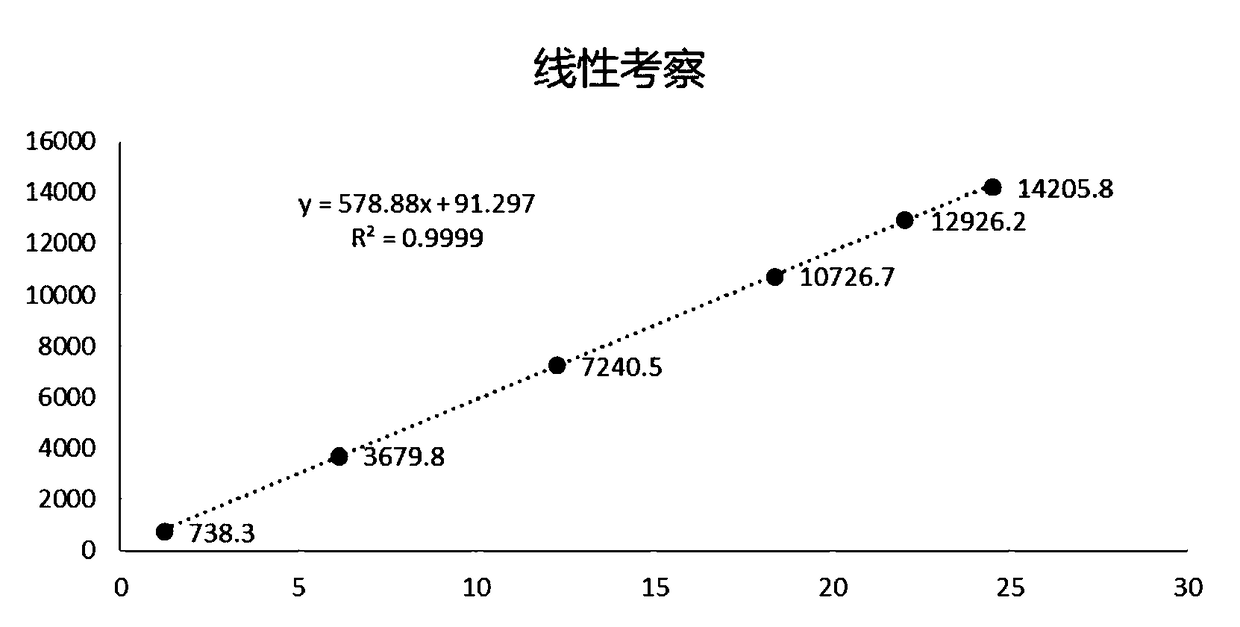
Method for determining 6-gingerol content in processing adjuvant ginger juice
InactiveCN108169389ASimple sample handlingGood reproducibilityComponent separationGinger RhizomeAdjuvant
The invention provides a method for determining 6-gingerol content in a processing adjuvant ginger juice. According to the invention, methanol is taken as an extract, a sample is prepared through two-time ultrasonic treatment, and is detected through high performance liquid chromatography, and the 6-gingerol content in the ginger juice can be calculated according to a regression equation being y=578.88x+91.297 and r=0.9999. The sample processing method is simple, the 6-gingerol content in a sample solution is stabilized in at least 24 hours, method reappearance is good, precision is high, andthe method is suitable for large-area popularization.
Owner:TIANSHENG PHARMA GROUP
Orientia tsutsugamushi nucleic acid fluorescence isothermal amplification primer, probe, kit and detection method
PendingCN113186304AExperimental hardware requirements are lowShort amplification timeMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionOrientia
The invention provides an orientia tsutsugamushi nucleic acid fluorescence isothermal amplification primer. The primer is screened from an Ot1 sequence, the primer comprises an upstream primer and a downstream primer, the upstream primer has the sequence characteristic of SEQ ID NO.1, and the downstream primer has the sequence characteristic of SEQ ID NO.2. The invention provides an orientia tsutsugamushi nucleic acid fluorescence isothermal amplification probe, the probe is screened from an Ot1 sequence, and the probe (Ot-P) has the sequence characteristic of SEQ ID NO.3. The invention discloses an orientia tsutsugamushi nucleic acid fluorescence isothermal amplification kit. The kit comprises the primer and the probe. The invention provides a detection method using the orientia tsutsugamushi nucleic acid fluorescence isothermal amplification kit. The detection method comprises the following steps: S1, treating a template; s2, treating the template, the freeze-dried enzyme powder, the primer, the probe, ultrapure water (Ultrapure water, UP water, the resistivity of which reaches 18 M omega.cm) and an activating agent, and reacting at the temperature of 35-41 DEG C for 20-40 minutes. The primer and the probe are high in specificity and sensitivity; the detection time is short, large expensive instruments and variable temperature systems are not needed, nucleic acid amplification can be completed at 37 DEG C within 30 minutes, and on-site rapid nucleic acid detection and popularization are easy.
Owner:HAINAN MEDICAL COLLEGE
Device for obtaining 3D biopsy
ActiveUS10463349B2Simple sample handlingEasy to determineSurgical needlesDiagnostics using spectroscopyBiopsy deviceGeneral surgery
A biopsy device for taking a 3D biopsy may comprise an outer sleeve, a hollow main shaft, a biopsy tube and a tube shaft. The hollow main shaft may have a distal end portion with a sideward facing notch, and the main shaft may be adapted to be accommodated within the outer sleeve. The biopsy tube may be provided for receiving cut and thus isolated tissue. A proximal end of the biopsy tube may be releasably attachable to a distal end of the tube shaft so that the biopsy tube is movable together with the tube shaft within the hollow main shaft between a proximal position in which the biopsy tube is not located in the notch, and a distal position in which the biopsy tube is located in the notch.
Owner:KONINKLJIJKE PHILIPS NV
Method and kit for simultaneously detecting vancomycin and norvancomycin in blood sample and application thereof
InactiveCN113049698ASimple sample handlingHigh sensitivityComponent separationTandem mass spectrometryFluid phase
The invention provides a method and a kit for simultaneously detecting vancomycin and norvancomycin in a blood sample and application of the method and the kit, and belongs to the technical field of medicine detection. The method for simultaneously detecting vancomycin and norvancomycin in the blood sample is successfully established by optimizing a sample pretreatment method and combining the sample pretreatment method with a high performance liquid chromatography-tandem mass spectrometry method, and has the advantages of simplicity in sample treatment, high sensitivity, high flux, reliable result and the like; the real-time monitoring on the blood level of the related antibiotics of the human body can be realized, so that the method has a good practical application value.
Owner:JINAN YING SHENG BIOTECH
Excrement metabolite for detecting curative effect of active pulmonary tuberculosis and detection system thereof
The invention discloses a method for judging whether an active pulmonary tuberculosis patient to be detected is cured or not after being treated for 6 months by an antituberculosis drug standard basedon an excrement LCMS metabonomics technology. The method comprises the following steps: carrying out LCMS (Liquid Chromatography Mass Spectrometry) detection on excrement of a to-be-detected patientto respectively obtain relative expression quantities of 14 differential metabolites, and judging whether the to-be-detected patient is cured or not according to the relative expression quantities ofthe 14 differential metabolites. Compared with the traditional method, the method provided by the invention has the advantages of non-invasion, simple sample treatment, no damage to the sample, smallsample injection amount, good repeatability, low cost and the like.
Owner:广东省结核病控制中心
Method for measuring content of lead and bismuth elements in depollution solution
InactiveCN105067590ASimple and efficient operationSimple sample handlingAnalysis by electrical excitationBismuth preparationInductance
The method for measuring the content of lead and bismuth elements in a depollution solution comprises steps as follows: (1) preparing a to-be-measured element standard solution; (2) preparing a sample solution I from the depollution solution before cleaning of parts for use; (3) preparing a sample solution II from the equivalent depollution solution after cleaning of the parts for use; (4) setting test conditions of inductive coupled plasma atomic emission spectrometry, measuring element content of deionized water, the to-be-measured element standard solution, the sample solution I and the sample solution II through an inductive coupled plasma atomic emission spectrometer; (5) recording measuring results, subtracting the lead element content of the sample solution I from the lead element content of the sample solution II, subtracting the bismuth element content of the sample solution I from the bismuth element content of the sample solution II, if the two results are up to the standard, judging the content to be qualified, and otherwise, judging the content to be unqualified. The analysis process is convenient to operate, the reagent consumption is low, and the working efficiency can be improved substantially.
Owner:AECC AVIATION POWER CO LTD
Antioxidant activity detection method for traditional Chinese medicine preparation for activating blood to resolve stasis, and application of the method
PendingCN108918437ASimple sample handlingFast analysisColor/spectral properties measurementsInternal standardMethanol
The invention discloses an antioxidant activity detection method for a traditional Chinese medicine preparation for activating blood to resolve stasis, which includes steps of: 1) ultrasonically extracting the traditional Chinese medicine preparation with a methanol solution to obtain a sample solution; 2) dissolving 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) in methanol to obtain a DPPH / methanol work solution; 3) mixing the sample solution, the DPPH / methanol work solution and methanol according to certain ratio to obtain a to-be-detected solution, a blank solution and a contrast solution,detecting the absorbancy of the solutions by means of a microplate reader, then calculating removal rate; 4) performing result correction with Vc being an internal standard substance, and expressing the antioxidant activity of the traditional Chinese medicine preparation by means of SR sample / SRVC. The invention also provides an application of the detection method for detecting the antioxidant activity of Xuefuzhuyu capsule.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
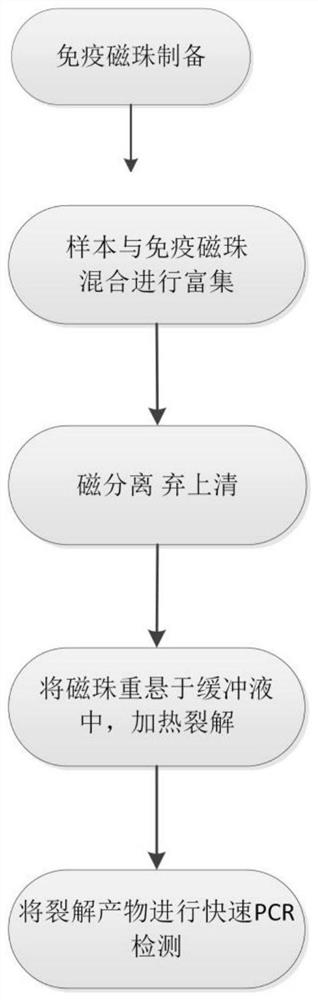
Hepatitis B virus enrichment fluorescent PCR (polymerase chain reaction) detection method
PendingCN114200127AEasy to storeEasy to transportMicrobiological testing/measurementBiological material analysisSuperparamagnetic beadsHepatitis B immunization
The invention relates to a hepatitis B virus enrichment fluorescent PCR (polymerase chain reaction) detection method which comprises the following steps: S1, preparation of immunomagnetic beads: coupling hepatitis B virus antibodies to carboxyl modified superparamagnetic beads to obtain the immunomagnetic beads coupled with the hepatitis B virus antibodies, s2, enrichment of hepatitis B virus: mixing and incubating hepatitis B virus positive serum or plasma with the immunomagnetic beads coupled with the hepatitis B virus antibody; s3, separating the immunomagnetic bead-virus compound by using a magnetic tool, resuspending the immunomagnetic bead-virus compound in a salt ion buffer solution, and heating and cracking; and S4, separating the magnetic beads by using a magnetic tool to obtain enriched and concentrated hepatitis B virus, and directly detecting liquid in a split product by using a fluorescent PCR reagent. According to the invention, the detection sensitivity is greatly improved due to enrichment and concentration, the specificity is strong due to immune recognition and gene recognition, and the fluorescent PCR detection time is short due to the use of a rapid PCR reagent.
Owner:杭州丹威生物科技有限公司
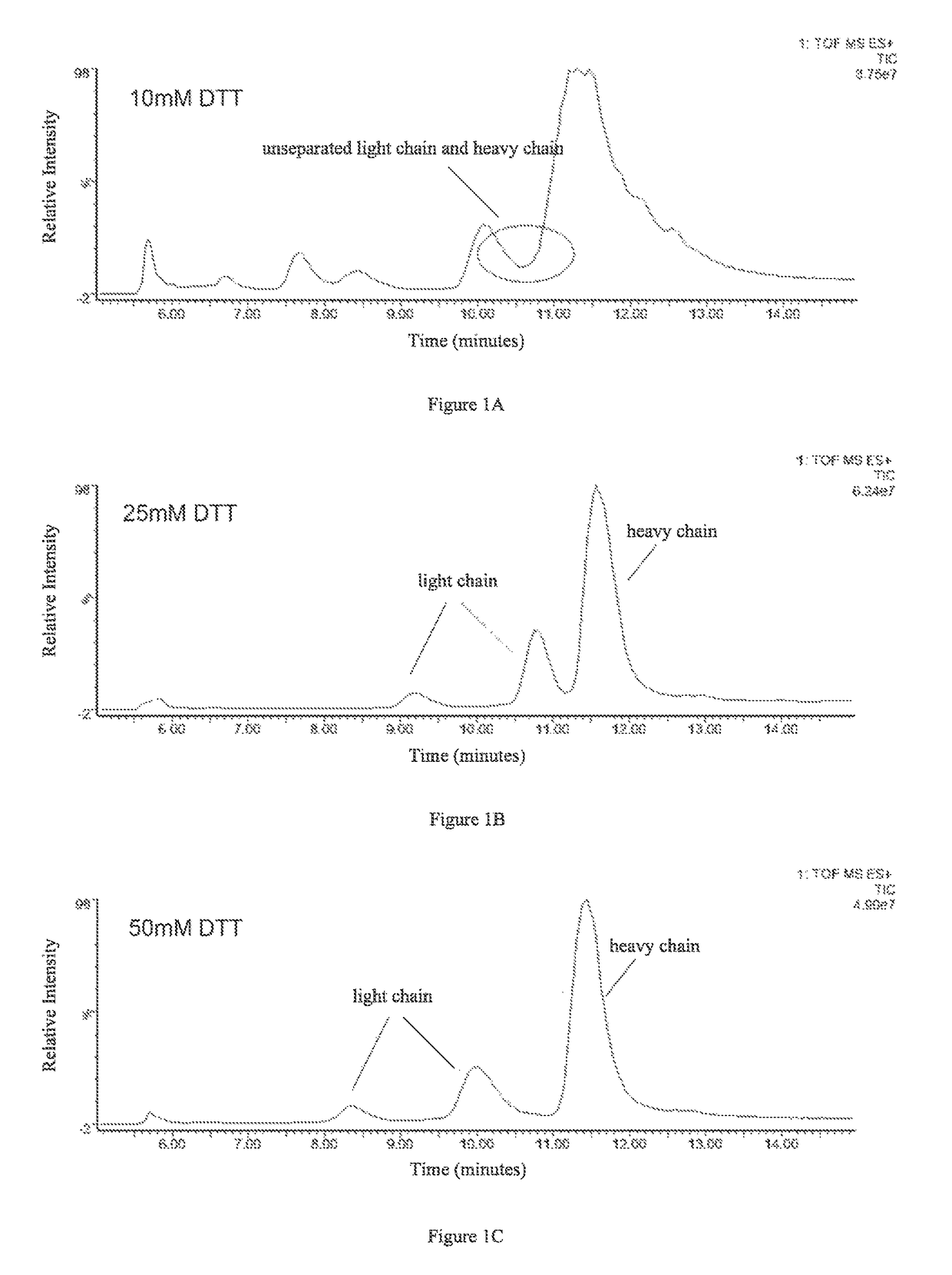
Method for determining glycosylation and terminal modification of samples during protein purification process
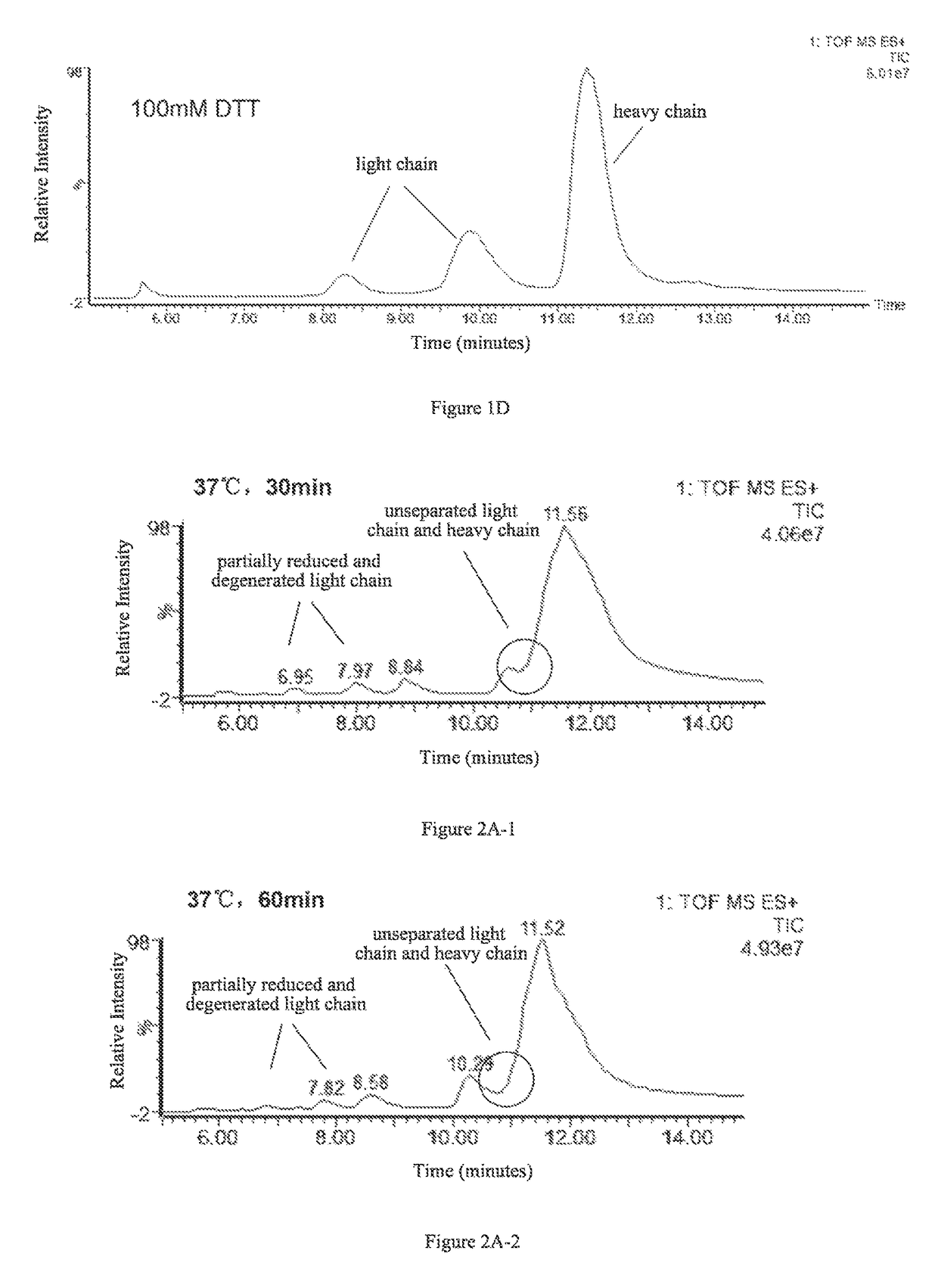
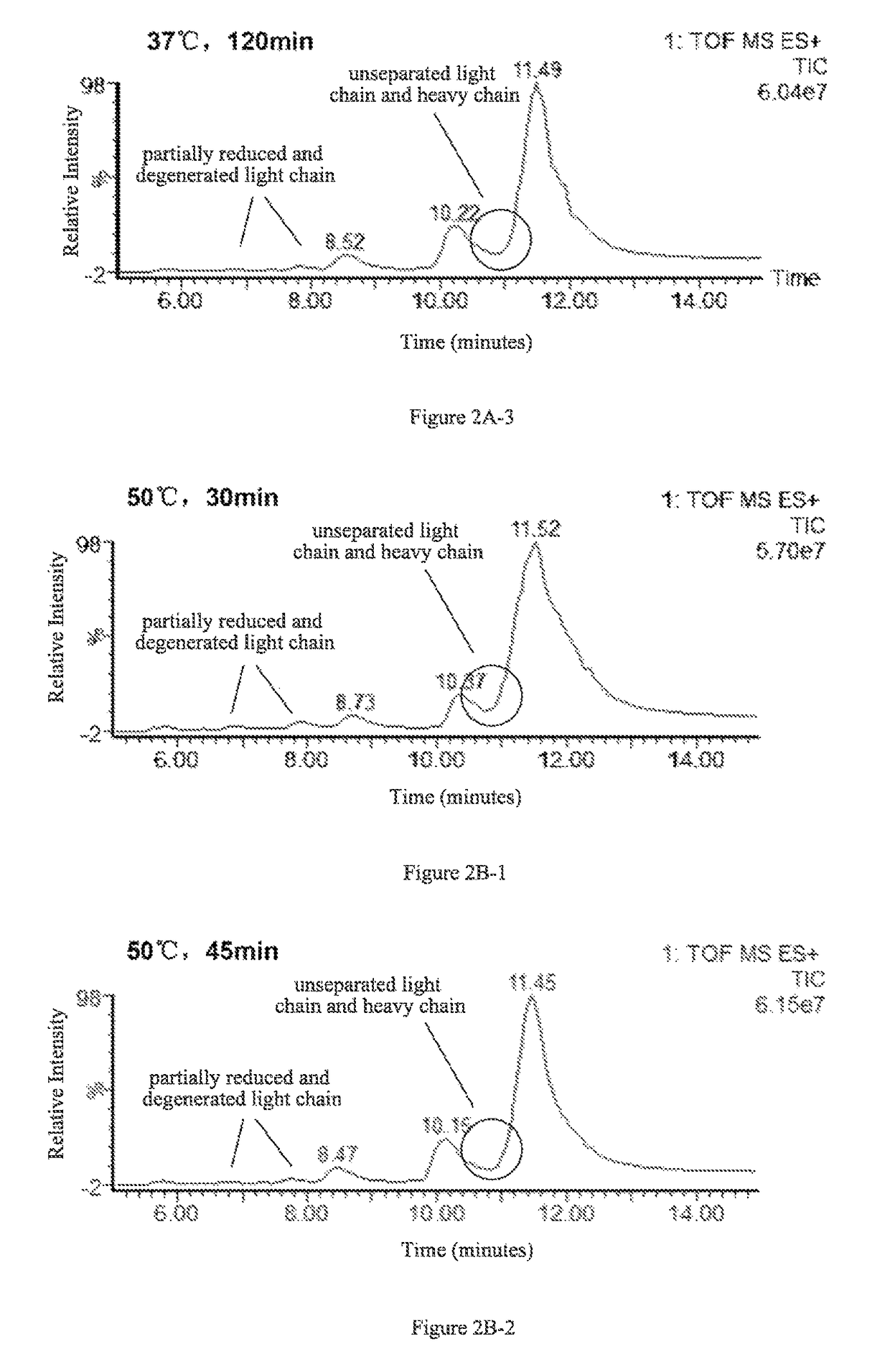
ActiveUS9645156B2Improve accuracyImprove resolutionComponent separationMass spectrometric analysisImmunglobulin eHeavy chain
The present invention provides a method for determining glycosylation and terminal modifications of immunoglobulin during immunoglobulin purification process, which can simultaneously and rapidly determine glycosylation, N-terminal pyroglutamination and C-terminal de-lysination of immunoglobulin, including Step 1) separating immunoglobulin by using cation-exchange resin, and collecting different components in according to retention time; Step 2) denaturing the components of immunoglobulin obtained in step 1) with a denaturant, followed by reducing them with a reducing agent, to separate the light chain and heavy chain; Step 3) separating the light chain and heavy chain of immunoglobulin of step 2) by using reverse phase ultrahigh pressure liquid chromatography; Step 4) measuring the molecular weights of the light chain and heavy chain obtained in step 3) with mass spectrum; and Step 5) analyzing the chromatographic data obtained in step 3) and the mass spectrometric data obtained in step 4) to determine glycosylation and terminal modifications of the immunoglobulin.
Owner:LIVZON MABPHARM
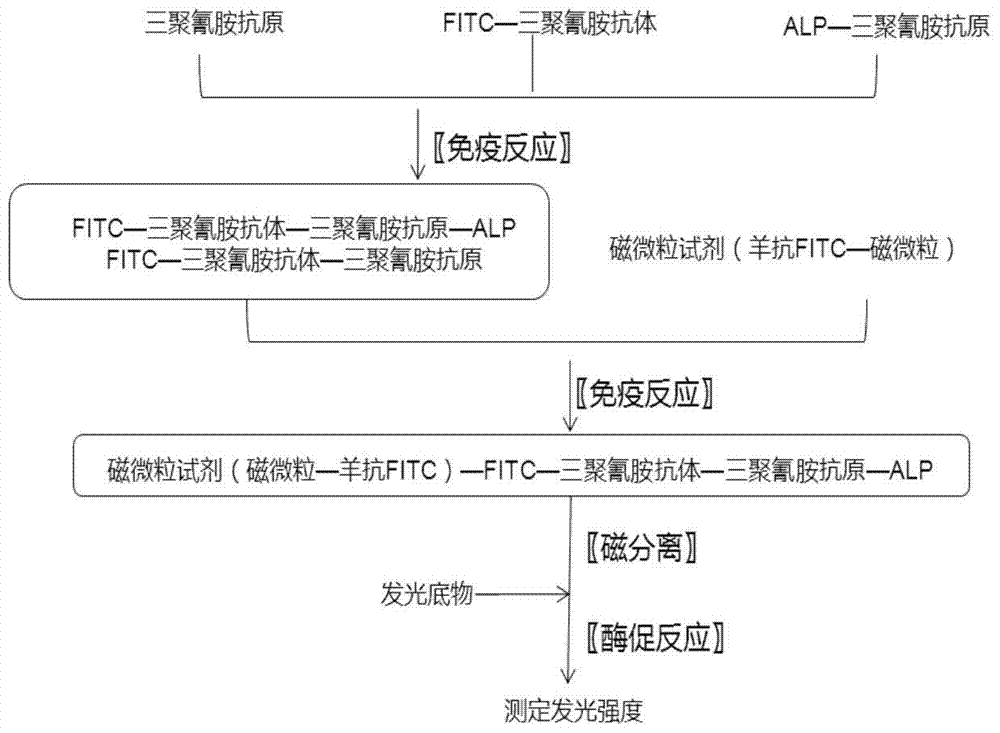
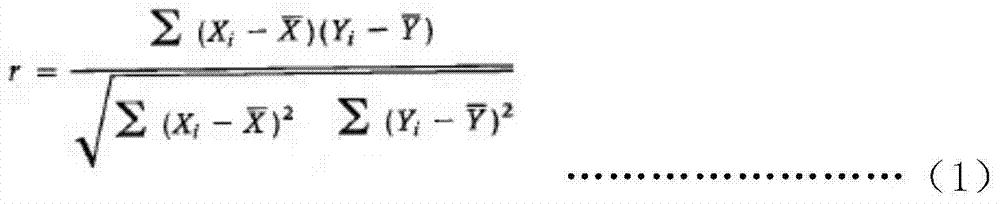
A magnetic bead separation chemiluminescent immunodetection method for melamine
ActiveCN105181680BSimple processing methodEasy to diluteChemiluminescene/bioluminescenceBiological testingMagnetic beadFluorescein isothiocyanate
Owner:CHENGDU CAPITALBIODX MEDICAL TECH CO LTD +1
A method for distinguishing tuberculous meningitis from viral meningitis based on nuclear magnetic resonance technology and its application
ActiveCN105943048BEasy to handleNon-destructiveDiagnostic recording/measuringSensorsMetaboliteNMR - Nuclear magnetic resonance
The invention discloses a method for distinguishing tubercular meningitis and virus meningitis based on a nuclear magnetic resonance technology and application thereof. According to the method, nuclear magnetic resonance detection is conducted on the cerebrospinal fluid of a patient to be detected to obtain relative expression quantities of difference metabolites represented by chemical shifts of 3.50 ppm, 3.76 ppm, 3.05 ppm, and 2.79 ppm respectively, and then whether the patient suffers from the tubercular meningitis or the virus meningitis is distinguished according to the relative expression quantities of the difference metabolites. Compared with the prior art, the method has the advantages of being simple in sample treatment, free of sample damage, small in sample quantity, good in repeatability, low in cost and the like.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV +1
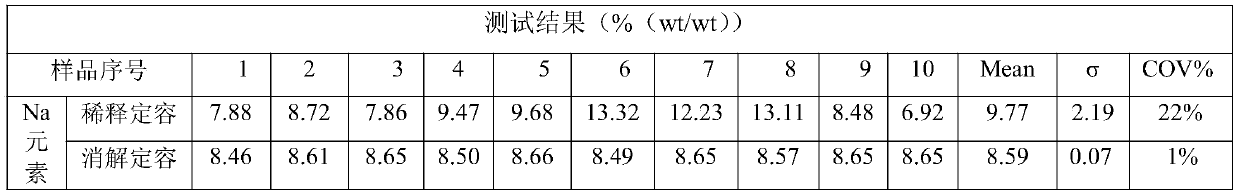
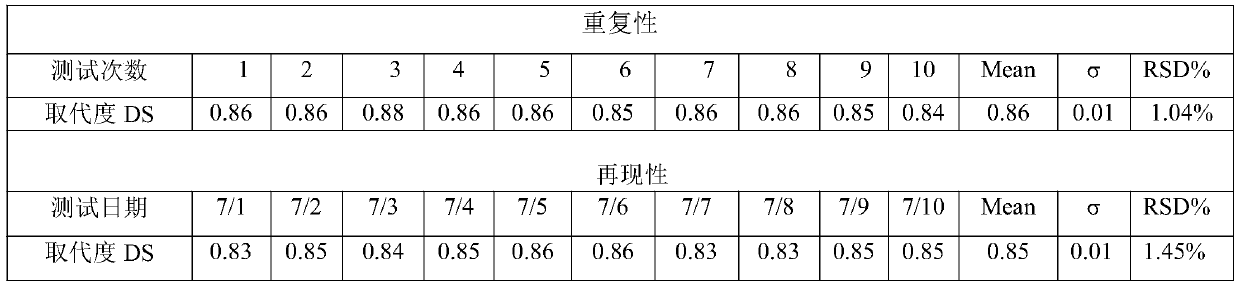
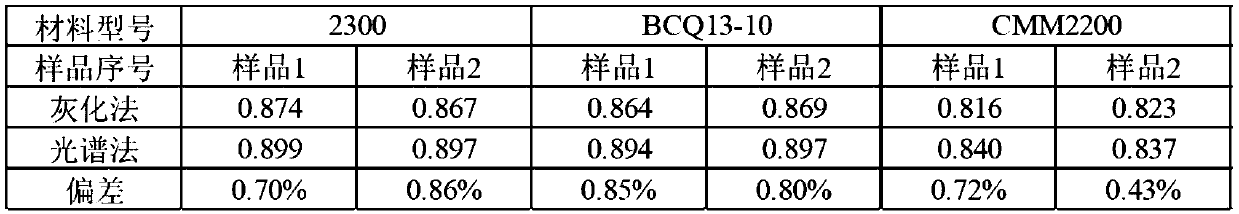
Method for testing substitution degree of sodium carboxymethylcellulose
InactiveCN110702668ALess samplesSimple sample handlingPreparing sample for investigationAnalysis by thermal excitationSodium carboxymethylcelluloseDigestion
The invention provides a method for testing substitution degree of sodium carboxymethylcellulose. The method comprises the following steps: S1, weighing a sample, putting the sample into a microwave digestion instrument for digestion, and after digestion is completed, transferring the sample into a volumetric flask for constant volume; S2, starting an ICP emission spectrometer, drawing a standardcurve, starting testing when the instrument is in a normal working state, and recording the content C of Na element; and S3, calculating the substitution degree DS of sodium carboxymethylcellulose according to the Na element content obtained by testing in the step S2, wherein DS is equal to 0.162B / (1-0.08B), and B is equal to (C-C0) * V / (m * MNa). Compared with the prior art, the method has the advantages that the sample is pretreated by adopting the microwave digestion instrument, the number of required samples is small, the sample treatment is simple, the digestion time is short, and the sample treatment time is shortened; and in addition, the content of sodium in CMC is detected by adopting the ICP emission spectrometer, the detected limit can be analyzed to be at the ppb level, and theaccuracy is high.
Owner:HUIZHOU LIWINON NEW ENERGY TECH CO LTD
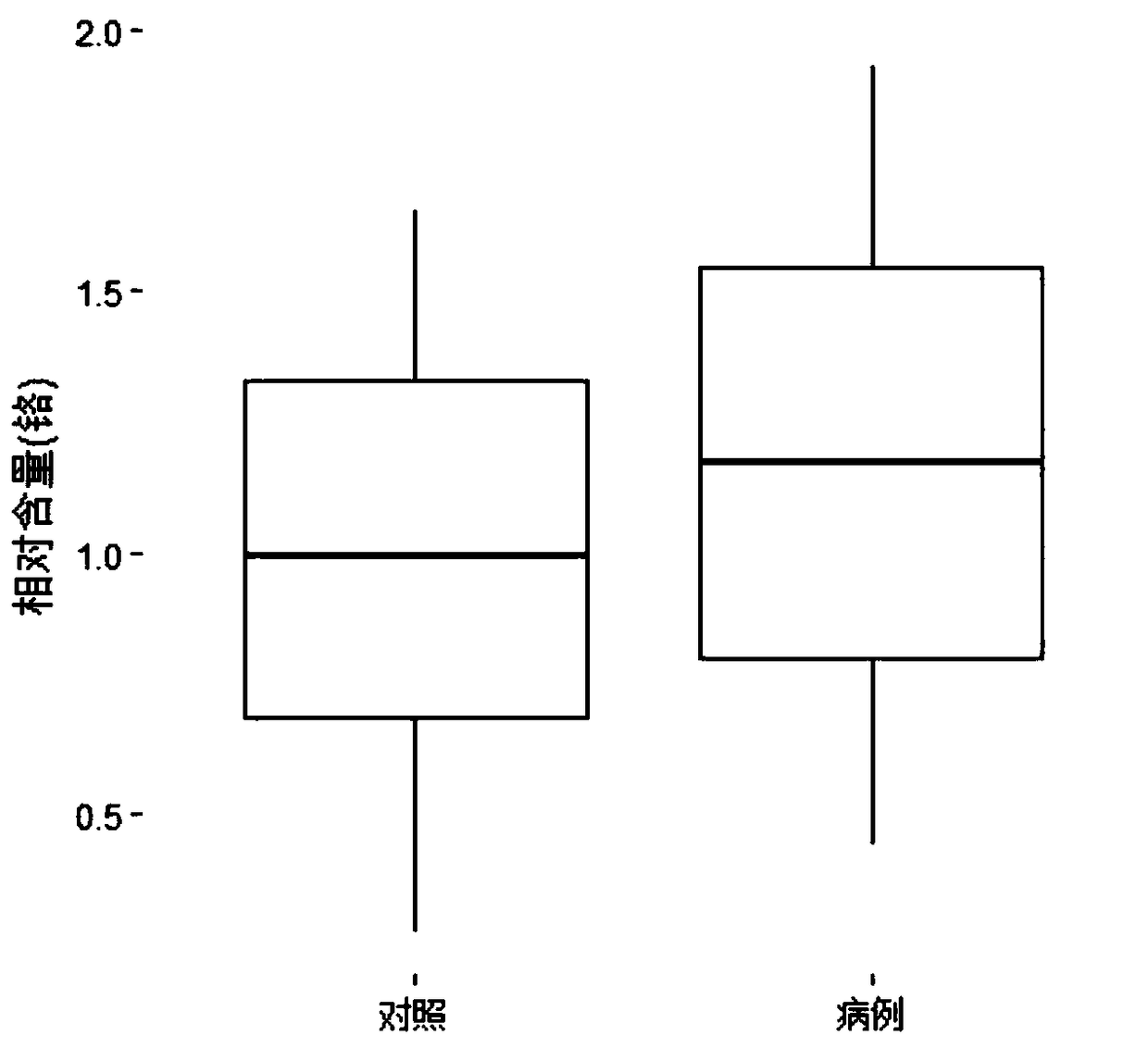
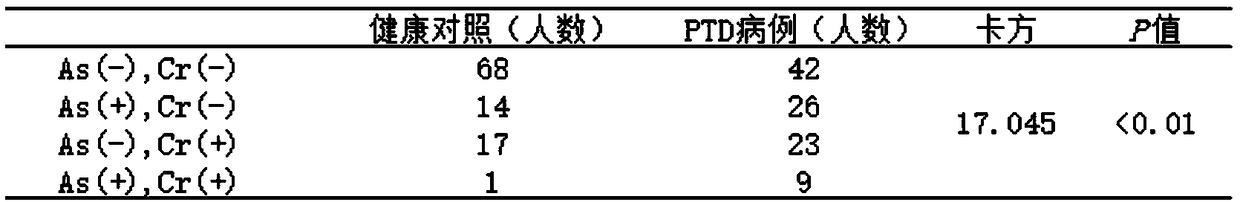
Auxiliary premature birth diagnosis marker based on mixed exposure detection of chromium and arsenic and application thereof
InactiveCN109164161ADelay and stop disease progressionSimple sample handlingMaterial analysis by electric/magnetic meansMixed exposureCHEMISTRY METHODS
The invention belongs to the field of analytical chemistry and clinical medicine, and discloses an auxiliary premature birth diagnosis marker based on mixed exposure detection of chromium and arsenicand application thereof. An exposure biomarker related to auxiliary premature birth diagnosis is based on mixed exposure of the chromium and the arsenic. The auxiliary premature birth diagnosis markerdetects the concentrations of the arsenic and the chromium in whole blood by ICP-MS, can be applied to auxiliary diagnosis of premature birth, is low in blood consumption and simple, fast and accurate in operation, and has a relatively good clinical popularization value.
Owner:NANJING MEDICAL UNIV
Method for detecting heavy metal pollution in paddy rice soil
InactiveCN110261336ASimple sample handlingEasy to operatePreparing sample for investigationColor/spectral properties measurementsWeedSpectrophotometry
The invention provides a method for detecting heavy metal pollution in paddy rice soil, and relates to the technical field of analysis and detection. The method comprises the following steps that 8 layers of soil are collected from the ground successively from top to bottom, the thickness of each layer is 3cm, 10g of each layer is taken randomly, and then the layers are mixed; the soil is fully crushed and air-dried, and is then collected after that impurities as stones and weeds are removed by sieving, 10g of the soil is taken and placed in a beaker, 20mL distilled water is added, and then a glass rod is used to carry out stirring to prepare a mixed solution a, a pH meter detects a pH value of the mixed solution, and 0.1mol / L NaOH solution is dropped in to adjust the pH value of the mixed solution a to 7.2-7.5; the mixed solution a is filtered to remove filter residues to obtain a mixed solution b, 2mL 10-32% of ammonia water is added into the mixed solution b, and a glass rod is used for stirring to prepare a mixed solution c; and heavy metal in the mixed solution c is detected in a atomic absorption spectrophotometry. The method of the invention is simple in operation and can improve the experimental efficiency.
Owner:JIANGSU TIANYU DETECTION TECH CO LTD
Lipid in excrement for detecting active pulmonary tuberculosis and detection system thereof
ActiveCN111537629ASimple sample handlingSample NoneComponent separationDisease diagnosisSimple samplePulmonary tb
The invention discloses an excrement metabolite for detecting active pulmonary tuberculosis and a detection system thereof. The excrement metabolite is selected from 13 kinds of differential metabolite; the detection system is based on an excrement LC-MS metabonomics technology, LC-MS detection is carried out on excrement of a to-be-detected patient, the relative expression quantities of 13 different metabolites are obtained respectively, and then whether a to-be-detected patient is an active pulmonary tuberculosis patient or not is judged according to the relative expression quantities of themetabolites. Compared with the traditional method, the method provided by the invention has the advantages of non-invasion, simple sample treatment, no damage to the sample, small sample injection amount, high repeatability, low cost and the like.
Owner:广东省结核病控制中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com