Synthesis method of nitrendipine metabolite
A technology for nitrendipine and a synthesis method, applied in the field of drug metabolism, can solve the problems of no such nitrendipine metabolite synthesis method, poor bioavailability, etc., and achieve great application research value, good technical effect, and operability. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 A kind of synthetic method of nitrendipine metabolite, it comprises the following steps:
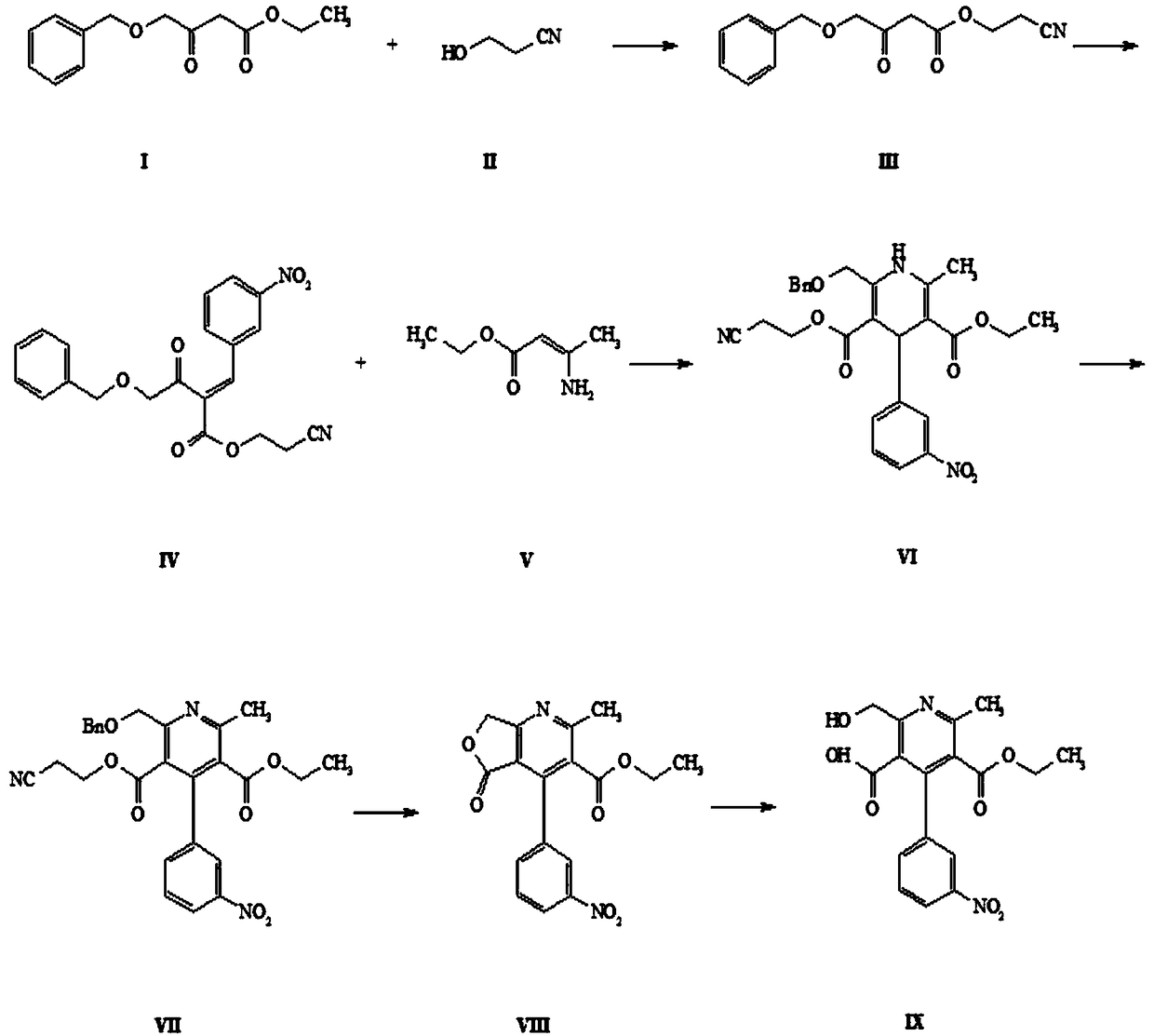
[0035] Such as figure 1 Shown is the reaction flow diagram of the present invention:
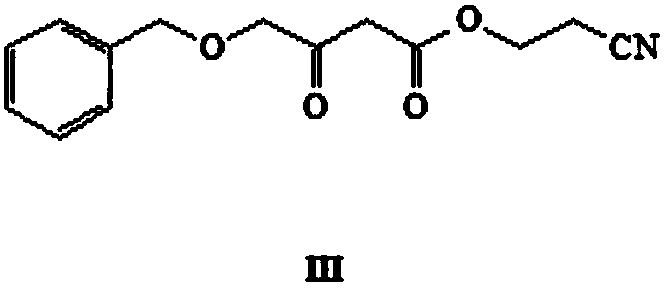
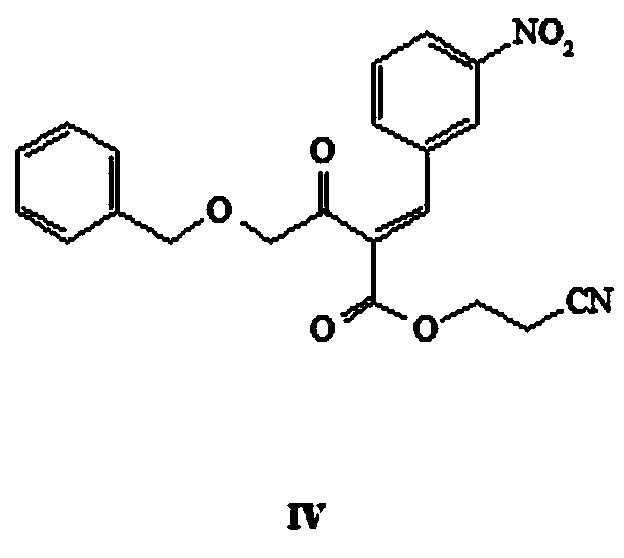
[0036] Step (1): Take a 1-liter flask and add 750 ml of toluene (newly opened, AR), add 35 g of 4-(benzyloxy)-3-oxobutanoic acid ethyl ester (I), add 70 mL of 3-hydroxypropionitrile (II), react at 130°C for 10 hours. TLC (sampling, DCM dilution plate), the reaction of raw materials is complete. The reaction mixture was cooled to room temperature, 200ml of water was added to the reaction solution, directly shaken and separated, the organic phase was concentrated by rotary evaporation at 40°C and then purified by column chromatography (developing solvent: petroleum ether: dichloromethane = 100:1), and the pure The mobile phase was concentrated to obtain 32.70g of intermediate III with a yield of 88.49%.
[0037] 1H NMR (600MHz, CDCl3) δ7.36(m,5H),4.60(s,2H),4.29(t,2H),4.14(s,3H),3...
Embodiment 2
[0053] The antihypertensive effect of embodiment 2 end product IX
[0054] The end product IX nitrendipine metabolites were used for antihypertensive experiments, and 60 hypertensive patients were selected, including 30 males and 30 females, aged 35 to 75 years, with an average age of 45 years. 60 hypertensive patients took the final product IX once a day, 10 mg once, and the total effective rate was 82%. Shows good potential antihypertensive effects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com