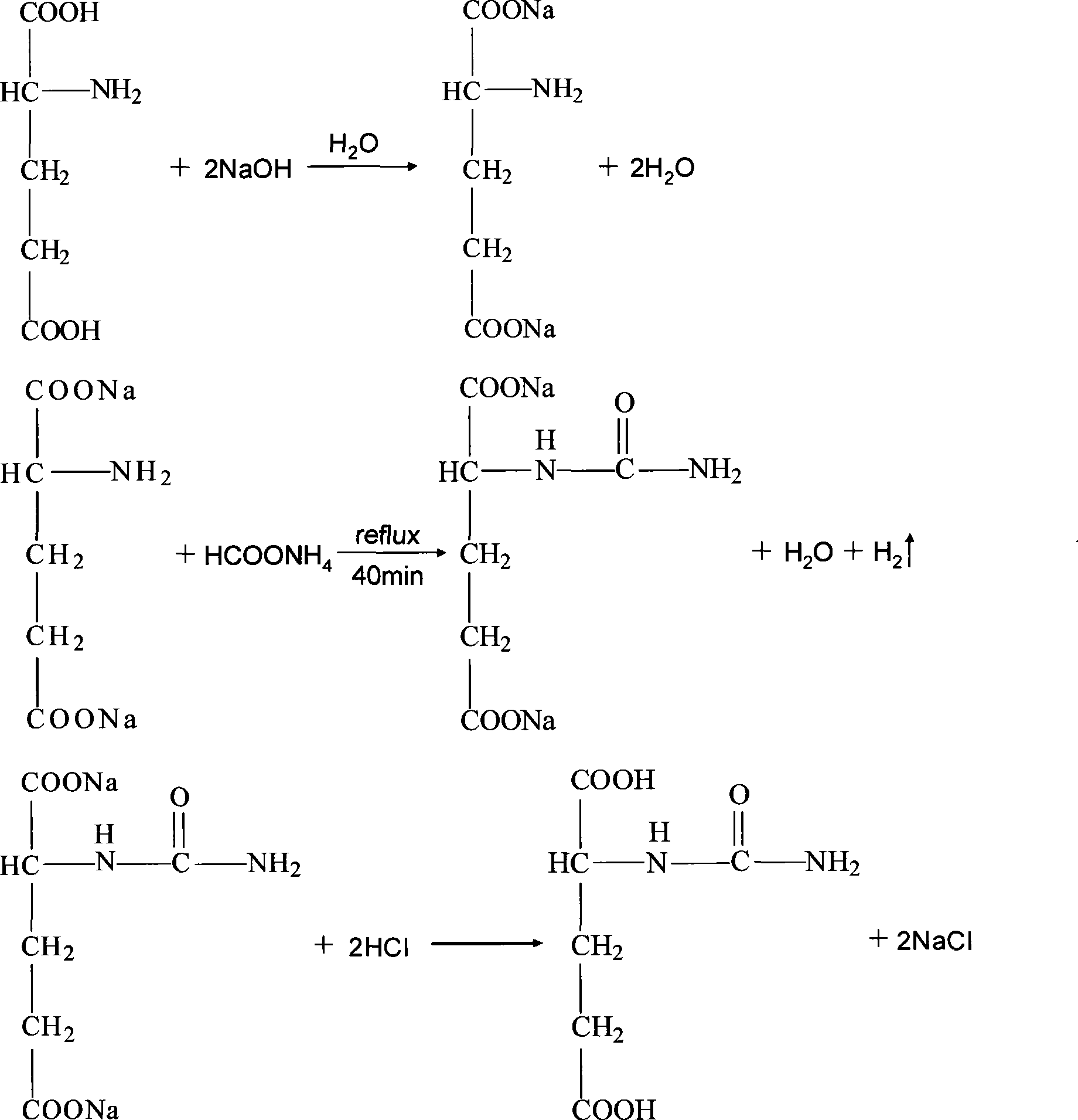
Preparation of N-carbamylglutamic
A technology of carbamoylglutamic acid and glutamic acid, applied in the field of preparation of N-carbamylglutamic acid, can solve the problem of unfavorable large-scale preparation of N-carbamylglutamic acid, and the chemical reaction equation is not fully disclosed. , long chemical reaction time and other problems, to achieve the effect of improving reproductive performance, increasing average weaning weight, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The method for preparing N-carbamyl glutamic acid comprises the following steps:
[0026] (1) Take glutamic acid 0.8mol, ammonium formate 0.8mol and sodium hydroxide 2.8mol for subsequent use;
[0027] (2) Dissolve the glutamic acid and sodium hydroxide taken in step (1) in 0.8L distilled water, stir, then add the ammonium formate taken in step (1) in the mixture obtained, fully stir, and mix the total The mixture was refluxed at 110°C for 30 minutes;
[0028] (3) Cool the reaction mixture obtained in step (2) to room temperature, then wash with formaldehyde, filter to obtain N-carbamoylglutamic acid sodium salt, add 1.6mol 30% concentrated hydrochloric acid to acidify, and then stand at -4°C N-carbamoylglutamic acid crystals were precipitated in 2 hours, and N-carbamoylglutamic acid was obtained by filtration with a yield of 78%.
Embodiment 2
[0030] The method for preparing N-carbamyl glutamic acid comprises the following steps:
[0031] (1) Take glutamic acid 1.6mol, ammonium formate 1.6mol and sodium hydroxide 2.5mol for subsequent use;
[0032] (2) Dissolve the glutamic acid and sodium hydroxide taken in step (1) in 1.5L distilled water, stir, then add the ammonium formate taken in step (1) in the mixture obtained, fully stir, and mix the total The mixture was refluxed at 98°C for 55 minutes;
[0033] (3) Cool the reaction mixture obtained in step (2) to room temperature, then wash with formaldehyde, filter to obtain N-carbamoylglutamic acid sodium salt, add 2.5mol 45% concentrated hydrochloric acid to acidify, and then stand at 2°C for 4.5 N-carbamoylglutamic acid crystals were precipitated within hours, and N-carbamoylglutamic acid was obtained by filtration with a yield of 72%.
Embodiment 3
[0035] (1) Take glutamic acid 1.4mol, ammonium formate 0.9mol and sodium hydroxide 1.6mol for subsequent use;
[0036] (2) Dissolve the glutamic acid and sodium hydroxide taken by step (1) in 1.6L distilled water, stir, then add the ammonium formate taken by step (1) in the mixture obtained, fully stir, and mix the total The mixture was refluxed at 106°C for 33 minutes;
[0037] (3) Cool the reaction mixture obtained in step (2) to room temperature, then wash with formaldehyde, filter to obtain N-carbamoylglutamic acid sodium salt, add 1.6mol33% concentrated hydrochloric acid to acidify, and then stand at -3°C for 2.5 N-carbamoylglutamic acid crystals were precipitated within hours, and N-carbamoylglutamic acid was obtained by filtration with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com