Enzyme-chemocatalysis racemization removing preparation method for L-glufosinate-ammonium
A technology of chemical catalysis and glufosinate-ammonium is applied in the field of enzymatic preparation of chiral pesticides to achieve the effect of reducing process cost, low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
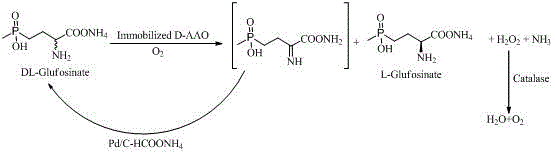
[0019] Add 20gDL-glufosinate-ammonium to 200mL ammonium formate (1mol / L, pH6.5) solution, stir to dissolve, add 2g immobilized D-amino acid oxidase (50u / g), 0.2mL catalase (50000u / mL) and 2g of palladium carbon (10%), continuously fed with oxygen, stirred and reacted at 30°C for 24h, and the D-enantiomer was completely reacted by chiral HPLC. The reaction solution was filtered, and the filtrate was adjusted to pH 8.0 with 28% ammonia water, concentrated under reduced pressure at 80°C to about 40 mL, cooled in an ice bath, and solids were precipitated, and dried to obtain 18 g L-glufosinate-ammonium with a yield of 90%, a purity of 98.4%, and ee99 .3%.
Embodiment 2
[0021] Add 20gDL-glufosinate-ammonium to 200mL1mol / L ammonium formate (pH6.5), stir to dissolve, add 4g immobilized D-amino acid oxidase (50u / g), 0.4mL catalase (50000u / mL) and 4g palladium carbon (10%), continuously fed with oxygen, stirred at 30°C for 12 hours, and the D-enantiomer was completely reacted by chiral HPLC. The reaction solution was filtered, and the filtrate was adjusted to pH 8.0 with 28% ammonia water, concentrated under reduced pressure at 80°C to about 40 mL, cooled in an ice bath, and solids were precipitated, and dried to obtain 19.6 g L-glufosinate-ammonium with a yield of 98% and a purity of 99.5%. ee99.8%.
Embodiment 3
[0023] Add 40gDL-glufosinate-ammonium to 200mL1mol / L ammonium formate (pH6.5), stir to dissolve, add 4g immobilized D-amino acid oxidase (50u / g), 0.4mL catalase (50000u / mL) and 4g palladium carbon (10%), continuously fed with oxygen, stirred at 30°C for 24 hours, and the D-enantiomer was completely reacted by chiral HPLC. The reaction solution was filtered, and the filtrate was adjusted to pH 8.0 with 28% ammonia water, concentrated under reduced pressure at 80°C to about 40 mL, cooled in an ice bath, and solids were precipitated, and dried to obtain 34 g L-glufosinate-ammonium with a yield of 85%, a purity of 96.3%, and ee98 .6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com