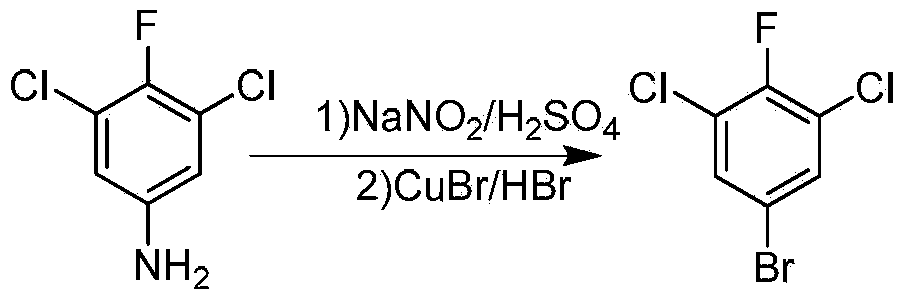Preparation method of 5-bromo-1,3-dichloro-2-fluorobenzene
A technology of fluorobromobenzene and dichloride, applied in the field of chemical intermediate preparation, can solve the problems of low reaction yield, unstable diazonium salt and the like, and achieve the effects of high purity, improved stability and yield, and easy mass preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 900g (5mol) of 3,5-dichloro-4-fluoroaniline and 1350ml of 98% concentrated sulfuric acid are used to form ammonium salt, and 1400g of 30% sodium nitrite aqueous solution are pumped into the tubular type with jacket with two metering pumps respectively. In the diazotization reactor (tube length 5m, inner diameter 7mm), tap water is passed through the jacket to adjust the temperature of the reaction solution in the tube to 15-20°C, the reaction solution stays in the tube for 15s, and then directly enters the bromine-filled tank with agitator. Put 715g (6mol) of cuprous chloride and 1000ml of 48% hydrobromic acid into a three-necked flask, and control the reaction temperature between 100 and 130°C. After the reaction is completed, cool down and extract twice with dimethane. After alkali washing and water washing, dichloromethane was distilled off to obtain crude 3,5-dichloro-4-fluorobromobenzene, which was rectified under reduced pressure to obtain 915g of 3,5-dichloro-4-fl...
Embodiment 2
[0030] Use 900g (5mol) of 3,5-dichloro-4-fluoroaniline and 1350ml of 98% concentrated sulfuric acid to make ammonium salt, and 1400g of 30% sodium nitrite aqueous solution into the jacketed pipe with two metering pumps respectively. In the type diazotization reactor (tube length 10m, inner diameter 7mm), tap water is passed through the jacket to adjust the temperature of the reaction solution in the tube to 15-20°C, the reaction solution stays in the tube for 30s, and directly enters the Put 715g (6mol) of cuprous bromide and 1000ml of 48% hydrobromic acid into a three-necked flask, control the reaction temperature between 100 and 130°C, after the reaction is completed, cool down, extract twice with dimethane, dichloromethane layer After washing with alkali and water, dichloromethane was distilled off to obtain crude 3,5-dichloro-4-fluorobromobenzene, which was rectified under reduced pressure to obtain 865 g of 3,5-dichloro-4-fluorobromobenzene (yield 71 %).
Embodiment 3
[0032] Use 900g (5mol) of 3,5-dichloro-4-fluoroaniline and 1350ml of 98% concentrated sulfuric acid to make ammonium salt, and 1400g of 30% sodium nitrite aqueous solution into the jacketed pipe with two metering pumps respectively. In the type diazotization reactor (the length of the pipe is 15m, the inner diameter is 7mm), tap water is passed through the jacket to adjust the temperature of the reaction liquid in the pipe to 15-20°C, the reaction liquid stays in the pipe for 60s, and directly enters the Put 715g (6mol) of cuprous bromide and 1000ml of 48% hydrobromic acid into a three-necked flask, control the reaction temperature between 100 and 130°C, after the reaction is completed, cool down, extract twice with dimethane, dichloromethane layer After washing with alkali and water, dichloromethane was distilled off to obtain crude 3,5-dichloro-4-fluorobromobenzene, which was rectified under reduced pressure to obtain 610 g of 3,5-dichloro-4-fluorobromobenzene (yield 50 %). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com