Preparation technology o high-purity gefitinib
A gefitinib and preparation process technology, applied in the field of pharmaceutical compound preparation, can solve the problems of many types of solvents, long production cycle, many steps, etc., and achieve the effects of high purity, reduced production cost, and reduced types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
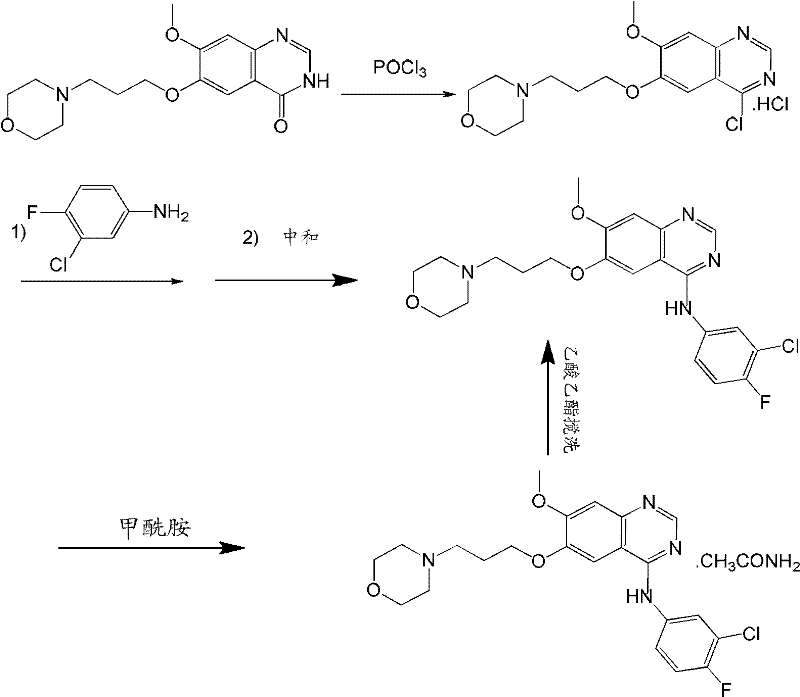
[0035] The preparation technology of high-purity gefitinib of the present invention is as follows:
[0036] 1: Add 10g 7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4(3H)-one (319.36g / mol) in a dry single-necked flask, 60ml three Phosphorus oxychloride, then add DMF 0.5ml dropwise, and heat to reflux in an oil bath at 110°C for 1h.
[0037] 2: The unreacted phosphorus oxychloride was evaporated under reduced pressure to obtain a viscous liquid.
[0038] 3: Add 6.3g (0.025mol) of 3-chloro-4-fluoroaniline and 80ml of isopropanol directly to the obtained viscous liquid without purification, heat and reflux in a water bath at 90°C for 6h, then stop heating and cool down for crystallization.
[0039] 4: Wash the material with cold isopropanol after filtering, and obtain the crude product of gefitinib hydrochloride after drying.
[0040] 5: Dissolve the crude product in hot water and adjust the pH to 8-9 with saturated sodium bicarbonate solution, then filter to obtain the crude...
Embodiment 2
[0045] The preparation technology of Gefitinib of the present invention is as follows:
[0046] 1: Add 60ml SOCl to a dry one-necked flask 2 , 0.5ml of DMF, then add 10g of 7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4(3H)-one (319.36g / mol), water bath 80°C Heat to reflux for 1h.
[0047] 2: Then evaporate the unreacted SOCl under reduced pressure 2 , to obtain a viscous liquid.
[0048] 3: Add 6.3g (0.025mol) of 3-chloro-4-fluoroaniline and 80ml of isopropanol directly to the obtained viscous liquid without purification, heat and reflux in a water bath at 90°C for 6h, then stop heating and cool down for crystallization.
[0049] 4: Wash the material with cold isopropanol after suction filtration, and obtain the crude product of gefitinib hydrochloride after drying.
[0050] 5: Dissolve the crude product in hot water and adjust the pH to 8-9 with saturated sodium bicarbonate solution, then filter to obtain the crude product of gefitinib.
[0051]6: Add crude gefitinib...
Embodiment 3
[0055] The preparation technology of Gefitinib of the present invention is as follows:
[0056] 1: Add 60ml SOCl to a dry one-necked flask 2 , 0.5ml of DMF, then add 10g of 7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4(3H)-one (319.36g / mol), water bath 80°C Heat to reflux for 1h.
[0057] 2: Then evaporate the unreacted SOCl under reduced pressure 2 , to obtain a viscous liquid.
[0058] 3: Add 6.3g (0.025mol) of 3-chloro-4-fluoroaniline and 80ml of isopropanol directly to the obtained viscous liquid without purification, heat and reflux in a water bath at 90°C for 6h, then stop heating and cool down for crystallization.
[0059] 4: Wash the material with cold isopropanol after suction filtration, and obtain the crude product of gefitinib hydrochloride after drying.
[0060] 5: Dissolve the crude product in hot water and adjust the pH to 8-9 with saturated sodium bicarbonate solution, then filter to obtain the crude product of gefitinib.
[0061] 6: Add crude gefitini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com