Gefitinib synthesis intermediate, and its preparing method and use
A use and compound technology, which is applied in the field of synthetic gefitinib intermediate compounds and its preparation, can solve problems such as increasing production costs, affecting product quality, and affecting yield, so as to improve quality, reduce two-step reactions, The effect of increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
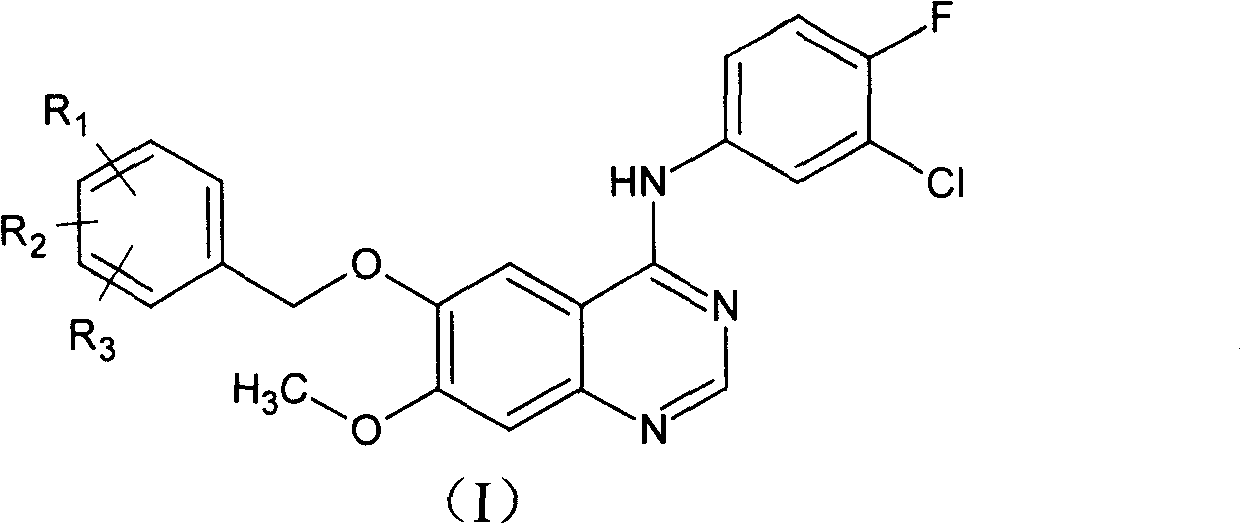
[0038] Example 1: Preparation of 6-benzyloxy-4-(3'-chloro-4'-fluoroaniline)-7-methoxyquinazoline
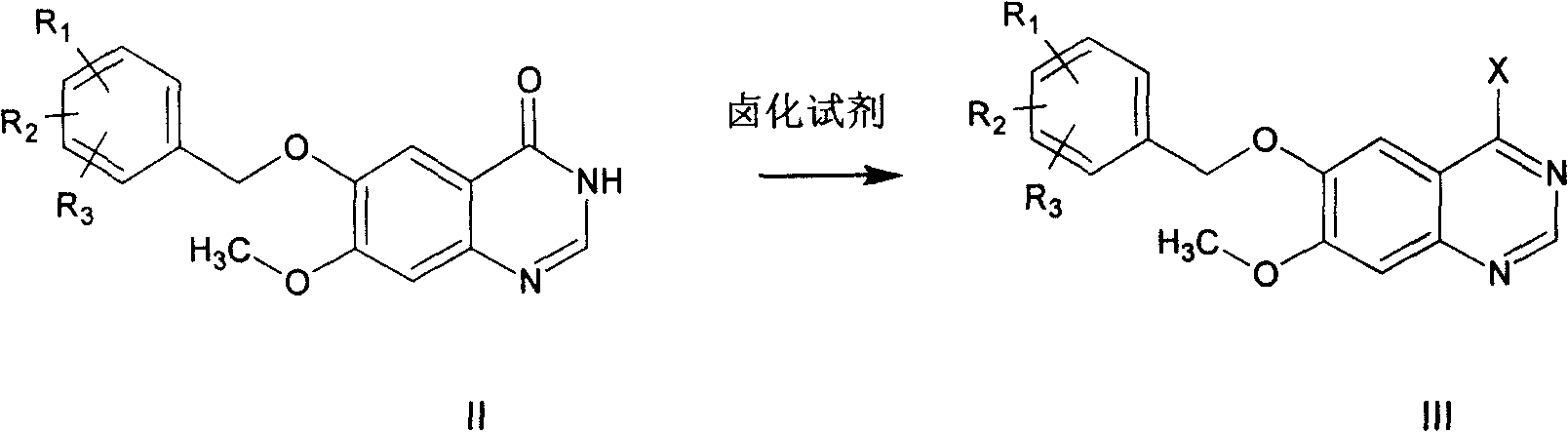
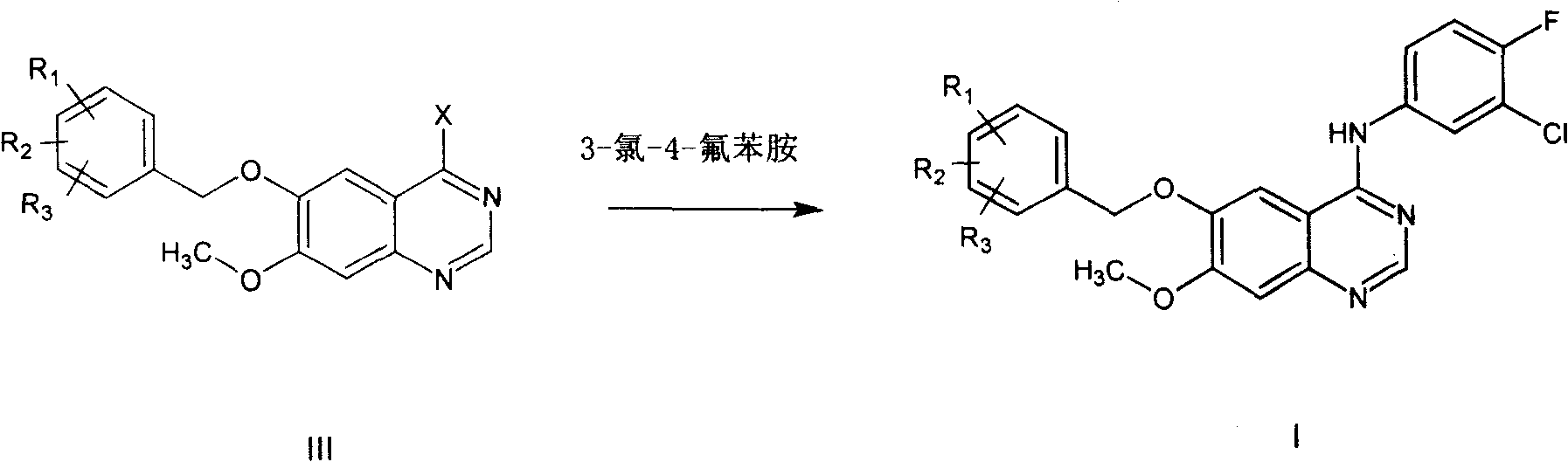
[0039] 6-Benzyloxy-7-methoxyquinazolinone (1.5g) was dissolved in 15mL SOCl 2 and 0.5mL DMF, heated to reflux for 5 hours, cooled, and evaporated SOCl under reduced pressure 2 , to obtain 6-benzyloxy-7-methoxy-4-chloroquinazolinone as an orange solid. Isopropanol (42ml) was added to the residue, and after dissolution, 3-chloro-4-fluoroaniline (0.9g) was added, heated to 90°C, and reacted for 3 hours. Cooled, filtered, and the precipitate was filtered off, washed once with methanol (2 ml), and dried to give 1.4 g (64%) of a white solid.
[0040] 1 H-NMR (DMSO): δ=3.99(s, 3H), 5.35(s, 2H), 7.37(s, 1H), 7.39-8.08(m, 8H), 8.62(s, 1H), 8.89(s, 1H).
Embodiment 2
[0041] Example 2: Preparation of 6-(2'-4'-dichloro)benzyloxy-7-methoxy-4-(3'-chloro-4'-fluoroaniline)quinazoline
[0042] According to the method of Example 1, take an equimolar amount of 6-(2'-4'-dichloro)benzyloxy-7-methoxyquinazolone instead of 6-benzyloxy-7-methoxyquinazolone For oxazolinone, operate in the same way to obtain 6-(2'-4'-dichloro)benzyloxy-7-methoxy-4-(3'-chloro-4'-fluoroaniline)quinazoline.
[0043] 1 H-NMR (DMSO): δ = 3.94 (s, 3H), 5.29 (s, 2H), 7.26 (s, 1H), 7.44-8.15 (m, 7H), 8.53 (s, 1H).
Embodiment 3
[0044] Example 3: Preparation of 6-(4'-methyl)benzyloxy-7-methoxy-4-(3'-chloro-4'-fluoroaniline)quinazoline
[0045] According to the method of embodiment 1, take the 6-(4'-methyl) benzyloxy-7-methoxyquinazolone of equimolar amount instead of 6-benzyloxy-7-methoxyquinazolone , and operated in the same way to obtain 6-(4'-methyl)benzyloxy-7-methoxy-4-(3'-chloro-4'-fluoroaniline)quinazoline.
[0046] 1H-NMR (DMSO): δ = 2.37 (s, 3H), 3.99 (s, 3H), 5.18 (s, 2H), 7.13-7.51 (m, 9H), 7.90 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com