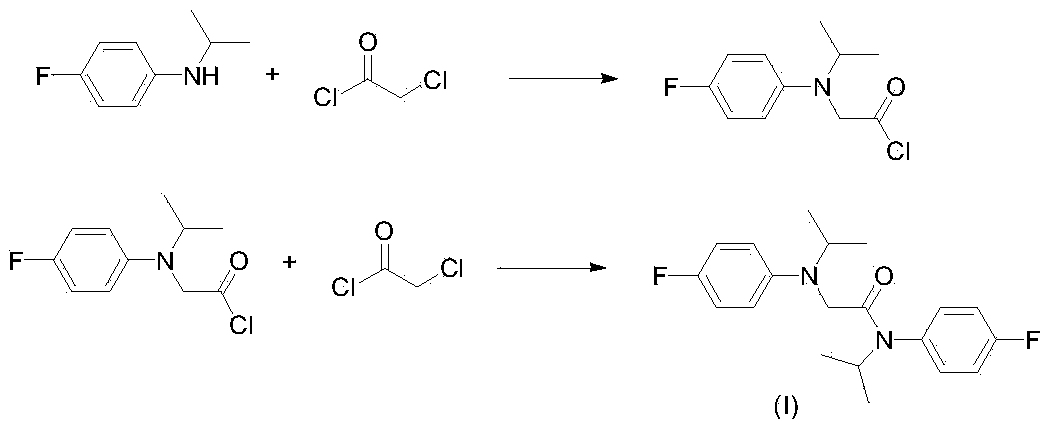
Method for preparing 2-chloro-N-(4-fluorophenyl)-N-isopropylacetamide
A technology of isopropylacetamide and fluorophenyl, which is applied in the field of preparation of 2-chloro-N--N-isopropylacetamide, can solve the problems of difficult removal, large amount of waste water, and high cost of raw material preparation, and achieves Easy to operate, less corrosive equipment, mild reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 154.7g of N-isopropyl-4-fluoroaniline, 102.2g of triethylamine and 400mL of toluene to a 1000mL three-necked flask equipped with mechanical stirring, a thermometer and a constant pressure dropping funnel, and add 114g of chloroacetyl chloride dropwise at room temperature , added dropwise for 1.5 hours, continued to react for 4 hours after dropping, added 150mL of water to wash, separated triethylamine hydrochloride aqueous solution, washed the organic phase with water, removed the solvent to obtain 228.7g of 2-chloro-N-(4-fluorobenzene base)-N-isopropylacetamide, purity 98.4%, yield 98%
[0018] 1 HNMR (400MHz, CDCl 3 ),&:7.16(m,2H),7.15(m,2H),4.97(m,1H),4.67(s,2H),1.07(d,J=5.2Hz,6H).
[0019] Add 40g sodium hydroxide in triethylamine hydrochloride aqueous solution, stir 10 minutes, separate liquid, organic layer adds 40g sodium hydroxide drying, after drying 12 hours, filter, obtain 97.3g triethylamine, recovery rate 95.2%, The sodium hydroxide filter cake is us...
Embodiment 2
[0021] Add 154.7g of N-isopropyl-4-fluoroaniline, 102.2g of triethylamine (97.3g from the previous batch) and 450mL of xylene to a 1000mL three-necked flask equipped with mechanical stirring, a thermometer and a constant pressure dropping funnel, Add 114g of chloroacetyl chloride dropwise at room temperature for 1.5 hours, continue to react for 4 hours after dropping, add 150mL of water to wash, separate triethylamine hydrochloride aqueous solution, wash the organic phase with water, and remove the solvent to obtain 228.5g of 2- Chloro-N-(4-fluorophenyl)-N-isopropylacetamide, purity 98.6%, yield 98.1%.
[0022] Add 40g of the previous batch of sodium hydroxide filter cake to triethylamine hydrochloride aqueous solution, stir for 10 minutes, separate liquids, add 40g of sodium hydroxide to the organic layer for drying, after drying for 12 hours, filter to obtain 98g of triethylamine, recover The rate is 95.9%, and the sodium hydroxide filter cake is used for the treatment of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com