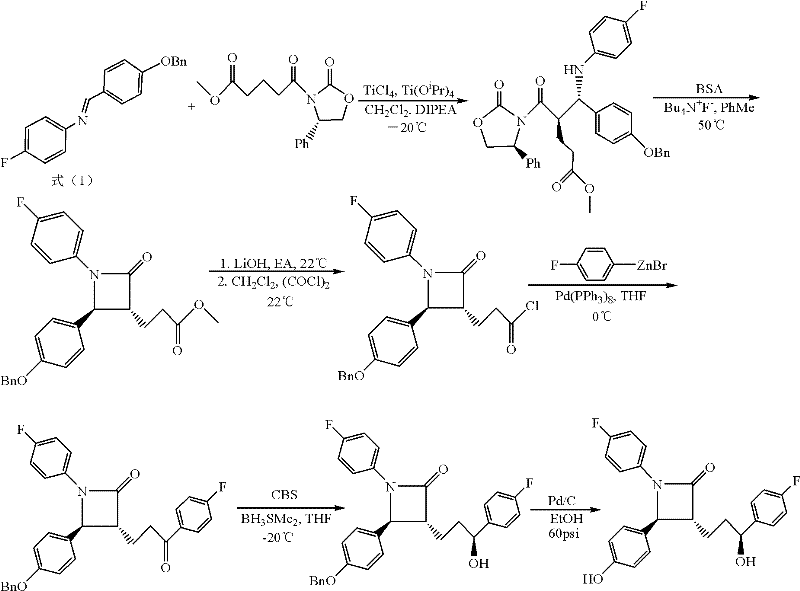
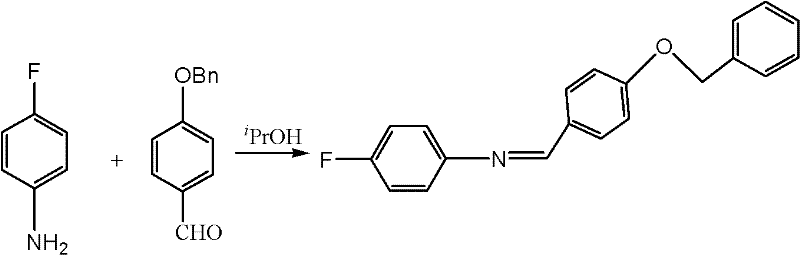
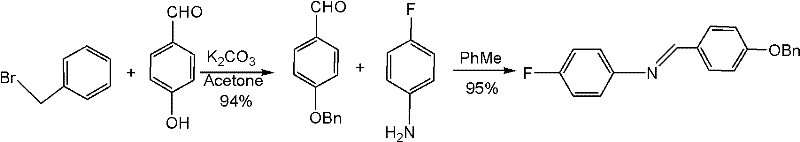
Preparation method of N-(4-fluorophenyl)-4-benzyloxy benzylidene amine
A technology of benzyloxybenzylidene and fluorophenyl, which is applied in the field of preparation of ezetimibe intermediate N--4-benzyloxybenzylidene, can solve the problem of high irritation and difficult operation of benzyl bromide , high price of benzyl bromide and other problems, to achieve the effect of simplifying the reaction operation steps, reducing the reaction cost, and reducing the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 4-hydroxybenzaldehyde (6g, 49.2mmol), 40ml of ethanol, potassium carbonate (7.5g, 54.3mmol), iodine (0.5g, 2mmol) and benzyl chloride (6.2ml, 54mmol) into a 100ml three-necked flask successively at room temperature ), heated to 80°C and refluxed for 3 hours, TLC detection (developing solvent: ethyl acetate / petroleum ether=2 / 5 (V / V)) was cooled to 45°C after the reaction was complete, and 4-fluoroaniline (5.6ml , 59mmol), while keeping the temperature below 55°C, solids precipitated out during the period, after dropping, continue to stir at 50°C for 2 hours, cool to room temperature, add 50ml of dichloromethane and 50ml of water to stir to dissolve the solids, separate layers, and reuse the water layer 20ml of dichloromethane was extracted once, the combined organic layers were washed with saturated Na 2 CO 3 Wash with 50ml×2 solution, 50ml×3 purified water, dry and concentrate to obtain a light yellow solid, add 20ml isopropanol and heat up to 45°C for slurry washi...
Embodiment 2
[0036]Add 4-hydroxybenzaldehyde (6g, 49.2mmol), ethanol 40ml, sodium carbonate (5.2g, 49.1mmol), sodium iodide (0.6g, 3.9mmol) and benzyl chloride (6.1 ml, 53mmol), heated to 60°C for 8 hours, TLC detection (developing solvent: ethyl acetate / petroleum ether = 2 / 5 (V / V)) after the reaction was complete, cooled to 45°C, slowly added dropwise 4-fluoroaniline (7.1ml, 74mmol), while keeping the temperature below 55°C, solids precipitated during the period, and continued to stir at 60°C for 4 hours after dropping, cooled to room temperature, added 50ml of dichloromethane and 50ml of water and stirred to dissolve the solids, layered, water layer was extracted once more with 20ml of dichloromethane, the combined organic layers were washed with saturated Na 2 CO 3 Wash with 50ml×2 solution, 50ml×3 purified water, dry and concentrate to obtain a light yellow solid, add 20ml isopropanol and heat up to 45°C for slurry washing for 1 hour, then cool to room temperature and filter, wash the...
Embodiment 3
[0038] Add 4-hydroxybenzaldehyde (6g, 49.2mmol), ethanol 60ml, potassium carbonate (10g, 73mmol), iodine (1g, 3.9mmol) and benzyl chloride (6.8ml, 59mmol) successively into a 100ml three-necked flask at room temperature, Heat up to 75°C for 7 hours, TLC detection (developing solvent: ethyl acetate / petroleum ether=2 / 5 (V / V)) After the reaction is complete, cool to 45°C, slowly add 4-fluoroaniline (7.1ml, 74mmol ), while keeping the temperature below 42°C, solids precipitated out during the period, and continued to stir at 42°C for 5 hours after dropping, cooled to room temperature and added 50ml of dichloromethane and 50ml of water to stir to dissolve the solids. Chloromethane was extracted once, the combined organic layers were washed with saturated Na 2 CO 3 Wash with 50ml×2 solution, 50ml×3 purified water, dry and concentrate to obtain a light yellow solid, add 20ml isopropanol and heat up to 45°C for slurry washing for 1 hour, then cool to room temperature and filter, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com