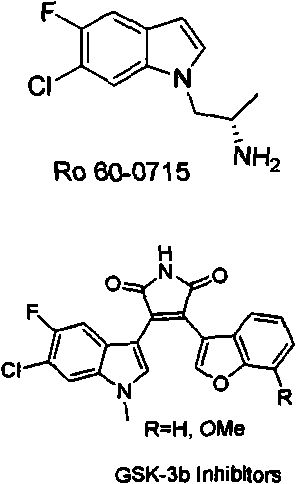
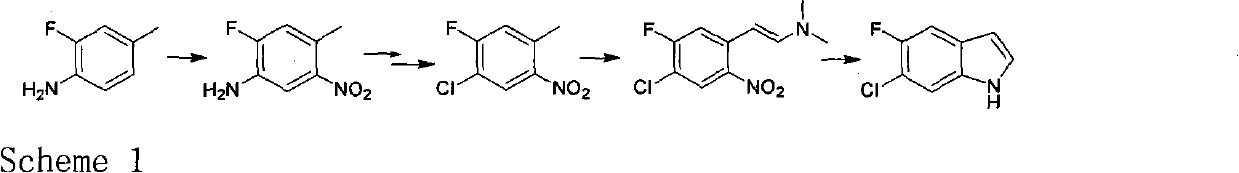
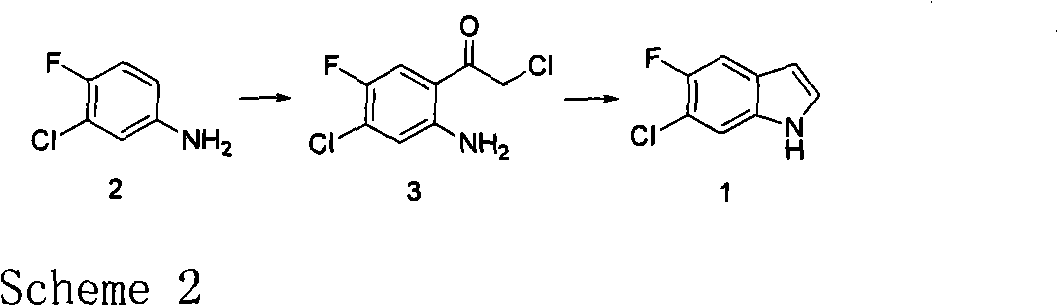
Novel method for preparing intermediate 6-chloro-5-fluoroindole used for synthesizing anticancer and weight-reducing medicine
An intermediate and new method technology, applied in the field of fluorine-containing indole as a pharmaceutical intermediate, can solve the problems of harsh reaction conditions, low yield, and large pollution in the synthesis process, and achieve the effect of short reaction steps, simple operation, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The first step reaction: preparation of intermediate 3
[0022] Add 4-fluoro-3-chloroaniline (7.28g, 5mmol) in toluene (60mL) dropwise to a solution of boron chloride (6.45g, 55mmol) in toluene (60mL) under an ice-water bath, and then to the mixed solution successively Chloroacetonitrile (3.8 mL, 60 mmol) and anhydrous aluminum trichloride (7.34 g, 55 mmol) were added, and the reaction solution was refluxed for 6-8 hours under nitrogen protection. After the reaction system was cooled, 2N hydrochloric acid was added thereto, and a large amount of precipitates precipitated out. Heat to 80°C with stirring until the solid dissolves. The reaction solution was extracted three times with dichloromethane after cooling. The aqueous phase was neutralized to neutral with 2N aqueous sodium hydroxide solution and extracted three times with dichloromethane. The organic phases were combined, dried, and the solvent was evaporated under pressure to obtain intermediate 3 (crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com