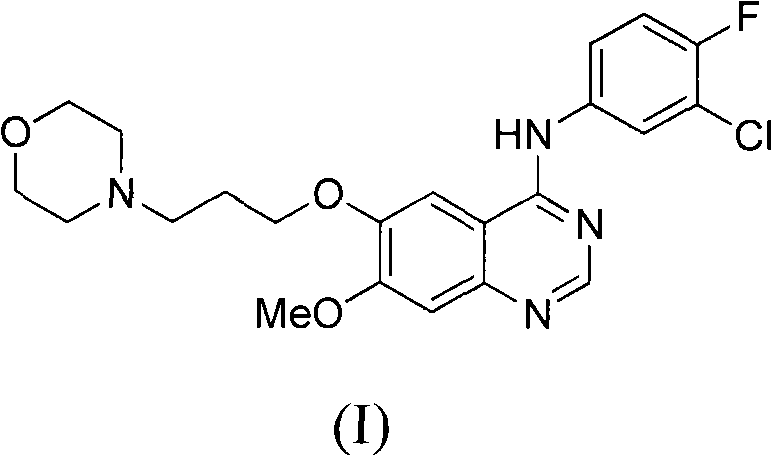
Novel method for preparing 4-(3-chlorine-4-fluorophenylamino)-7-methoxyl-6-(3-morpholinepropoxy)quinazoline
The technology of morpholine propoxy and fluorophenyl amine group is applied in the field of preparation of 4--7-methoxy-6-quinazoline, and can solve the problems of purification of untargeted products, decreased yield, and easy raw material amidines. decomposition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
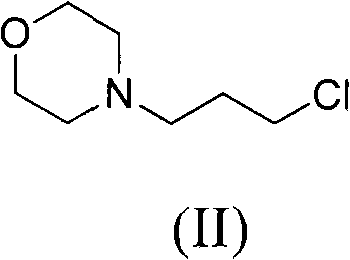
[0062] Example 1: Synthesis of 3-morpholinopropyl chloride (II)
[0063] Dissolve 193 g of morpholine in 580 ml of toluene and raise the temperature to 85°C. 159 grams of 1-bromo-3-chloropropane was slowly dropped into the above mixture, and stirred at 85°C for 1 day.
[0064] Under an ice bath, 500 ml of 18% hydrochloric acid aqueous solution was slowly added dropwise to the reaction solution, and after thorough stirring, the water layer was separated. Also under an ice bath, slowly drop 250 ml of 50% sodium hydroxide aqueous solution into the water layer. The water layer was extracted 3 times with ethyl acetate, and then the water layer was removed. After the organic layer was dried over anhydrous sodium sulfate, the organic solvent was removed under reduced pressure to obtain 147 g of product with a yield of 90%.
Embodiment 2
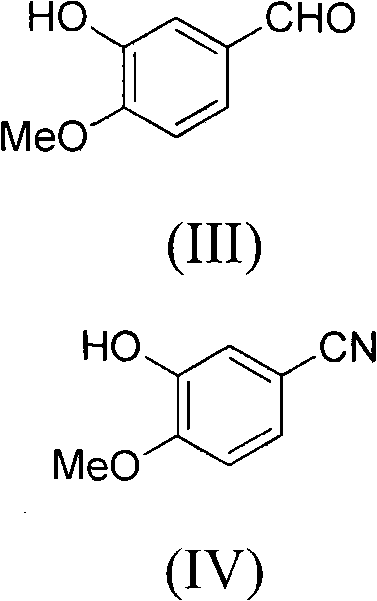
[0065] Example 2 Synthesis of 2-hydroxy-3-methoxybenzonitrile (IV)
[0066] 150 grams of 3-hydroxy-4-methoxybenzaldehyde (III) and 205 grams of sodium formate were dissolved in 1.5 liters of formic acid. When the system was heated to 85°C, 97 g of hydroxylamine sulfate was slowly added to the above mixed solution within 6 hours, and then reacted for 6 hours.
[0067] After cooling to room temperature, 5 liters of saturated brine was added to the reaction system, and the solid was filtered out. After drying, 145 g of white solid was obtained with a yield of 99%.
Embodiment 3
[0068] Example 3 Synthesis of 3-(3-morpholinopropoxy)-4-methoxybenzonitrile (V)
[0069] 89 grams of 2-hydroxy-3-methoxybenzonitrile (IV), 108 grams of 3-morpholinopropyl chloride (II), and 141 grams of potassium carbonate were dissolved in 1 liter of DMF. After heating to 75°C, react for 4 hours.
[0070] 1.5 liters of water was added to the reaction mixture, and extraction was performed 3 times with 2 liters of ethyl acetate. After the organic layer was dried over anhydrous sodium sulfate, the organic solvent was removed to obtain 165 g of viscous liquid with a yield of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com