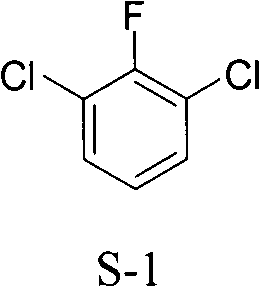
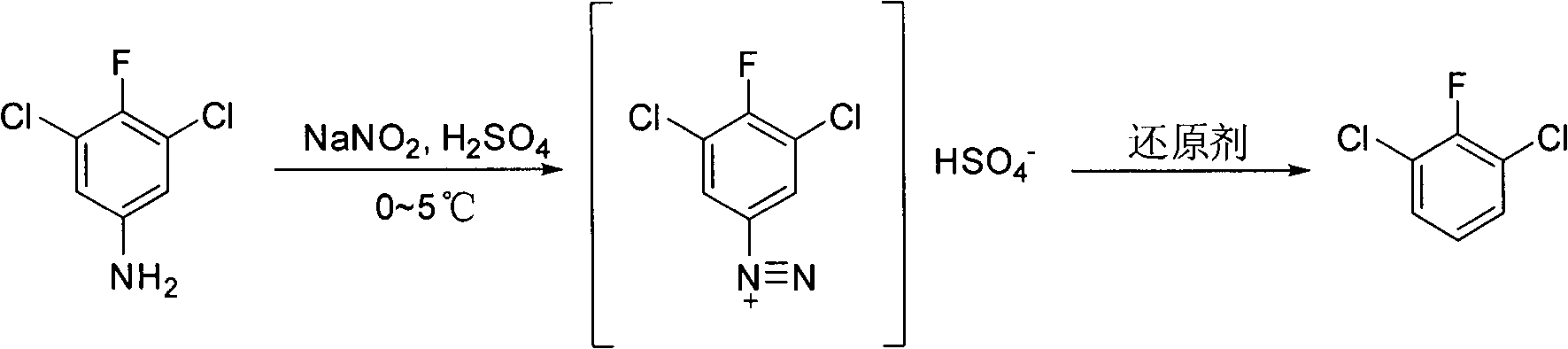
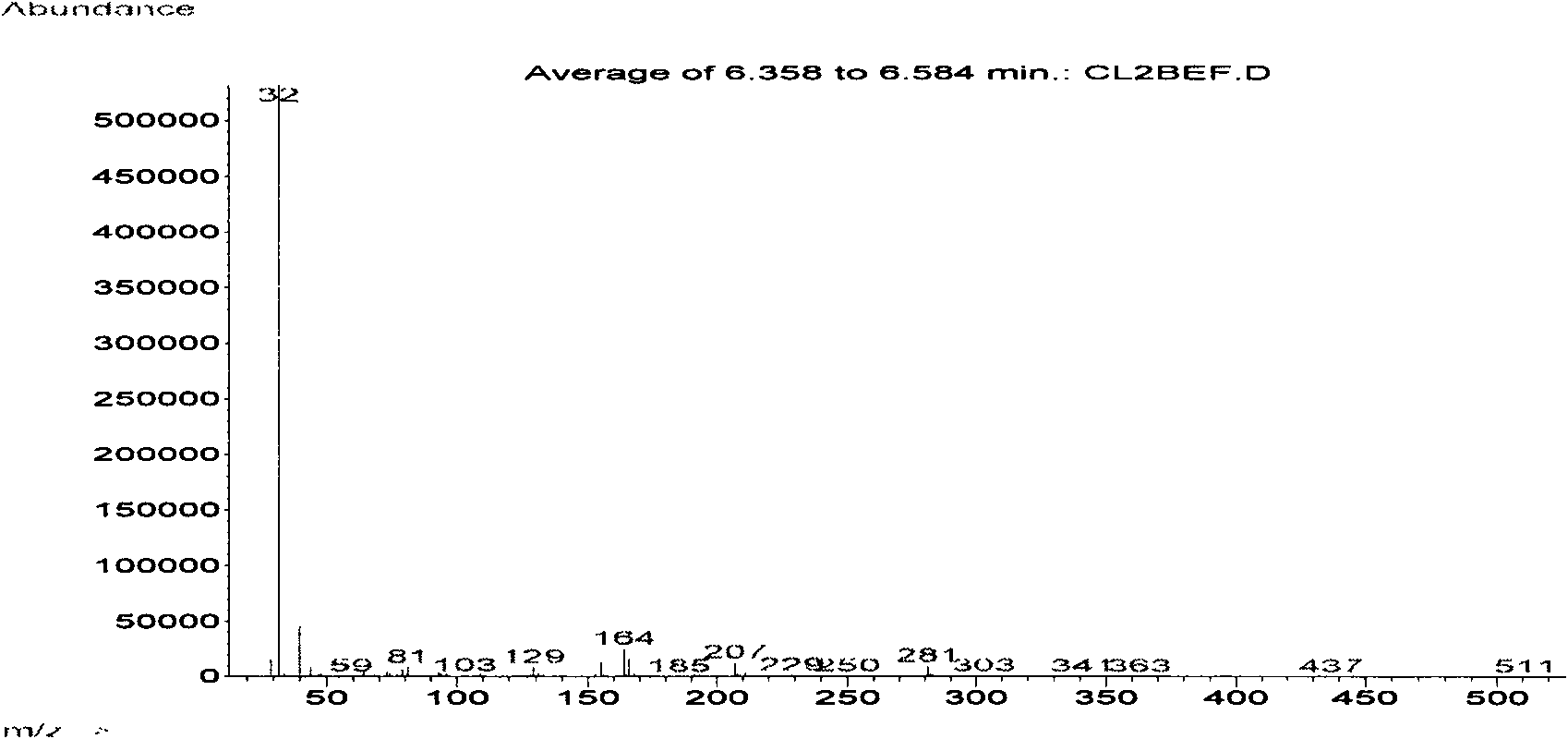Preparation method of 2, 6-dichlor fluorbenzene
A technology for dichlorofluorobenzene and dichloride, which is applied to the synthesis field of organic compounds, can solve the problems of difficult control of process conditions, complicated process, low product yield, etc., and achieves easy control of process conditions, wide sources, and reduced production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、3
[0027] Embodiment 1, the preparation method of 3,5-dichloro-4-fluoroaniline, with 3,5-dichloro-4-fluoronitrobenzene as raw material, the following steps are carried out successively:
[0028] (1) Dissolve 105g of 3,5-dichloro-4-fluoronitrobenzene in 200mL of ethanol, stir evenly, there is a strong endothermic phenomenon. Pour 70g of iron powder and 150g of ammonium chloride into a 1L three-necked flask, add 250mL of water to mix, start heating, and start adding the ethanol solution of the reactant dropwise when it reaches 60°C, and the dropwise addition is completed in about 90 minutes. At this time, the temperature is 78°C. When the reaction solution starts to reflux, the temperature is 94°C, and heat and keep warm.
[0029] (2) After reacting for 2 hours, the reaction liquid was cooled to room temperature, and 200 mL of toluene was added for extraction. Suction filtration to remove iron sludge, wash the filtrate with water, and filter to remove the precipitated Fe(OH)x prec...
Embodiment 2
[0030] Embodiment 2, a kind of preparation method of 2,6-dichlorofluorobenzene, take 3,5-dichloro-4-fluoroaniline as starting material, carry out the following steps successively:
[0031] (1) Concentrated H with a mass fraction of 98% 2 SO 4 6g (0.06mol) was diluted with water to 30g, added to a 250mL three-necked flask, and 3.6g (0.02mol) of 3,5-dichloro-4-fluoroaniline was added in batches in an ice bath, and reacted at room temperature for 0.7h. The reaction solution ( That is, the temperature of the solution containing ammonium salt) drops to 0°C; then drop 20mL of an aqueous solution containing 1.50g (0.021mol) of sodium nitrite, and react at 0-5°C for 1 hour, then use starch potassium iodide test paper to detect the end point, and the test paper turns blue , stop responding.
[0032] (2) Slowly add 3.5g (0.04mol) of ethyl acetate dropwise into the solution containing diazonium salt prepared in the above step (1), heat up to 70°C and reflux, and the nitrogen gas genera...
Embodiment 3
[0034] Embodiment 3, a kind of preparation method of 2,6-dichlorofluorobenzene, take 3,5-dichloro-4-fluoroaniline as starting material, carry out the following steps successively:
[0035] (1) Concentrated H with a mass fraction of 98% 2 SO 4 4g (0.04mol) was diluted with water to 20g, added to a 250mL three-necked flask, and 3.6g (0.02mol) of 3,5-dichloro-4-fluoroaniline was added in batches in an ice bath, and reacted at room temperature for 0.5h. Lower to -5°C; then drop into 20mL of aqueous solution containing 1.66g (0.024mol) of sodium nitrite, react at -5~0°C for 1 hour, use starch potassium iodide test paper to detect the end point, the test paper turns blue, and the reaction is stopped.
[0036] (2) Slowly add 3.5g (0.04mol) of ethyl acetate dropwise into the solution containing diazonium salt prepared in the above step (1), heat up to 70°C and reflux, and the nitrogen gas generated by the decomposition of diazonium salt will continue to form bubbles The form escapes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com