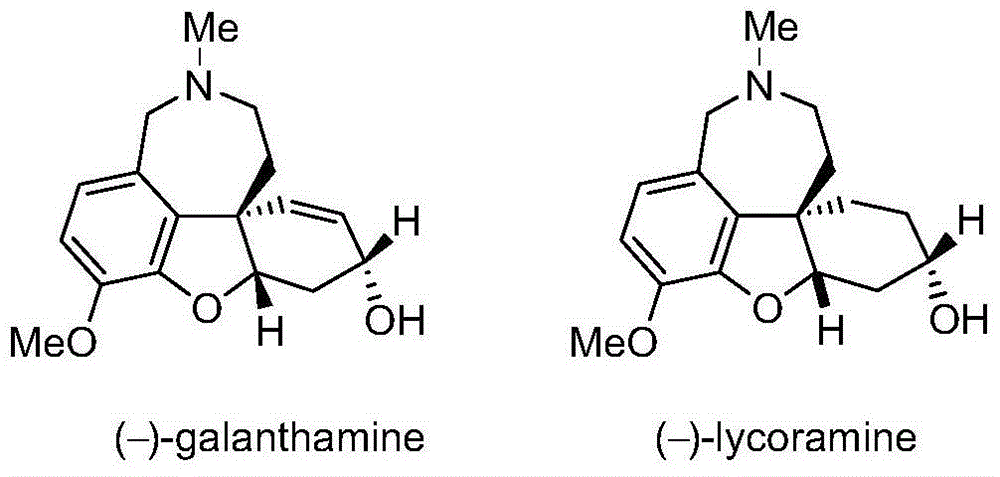
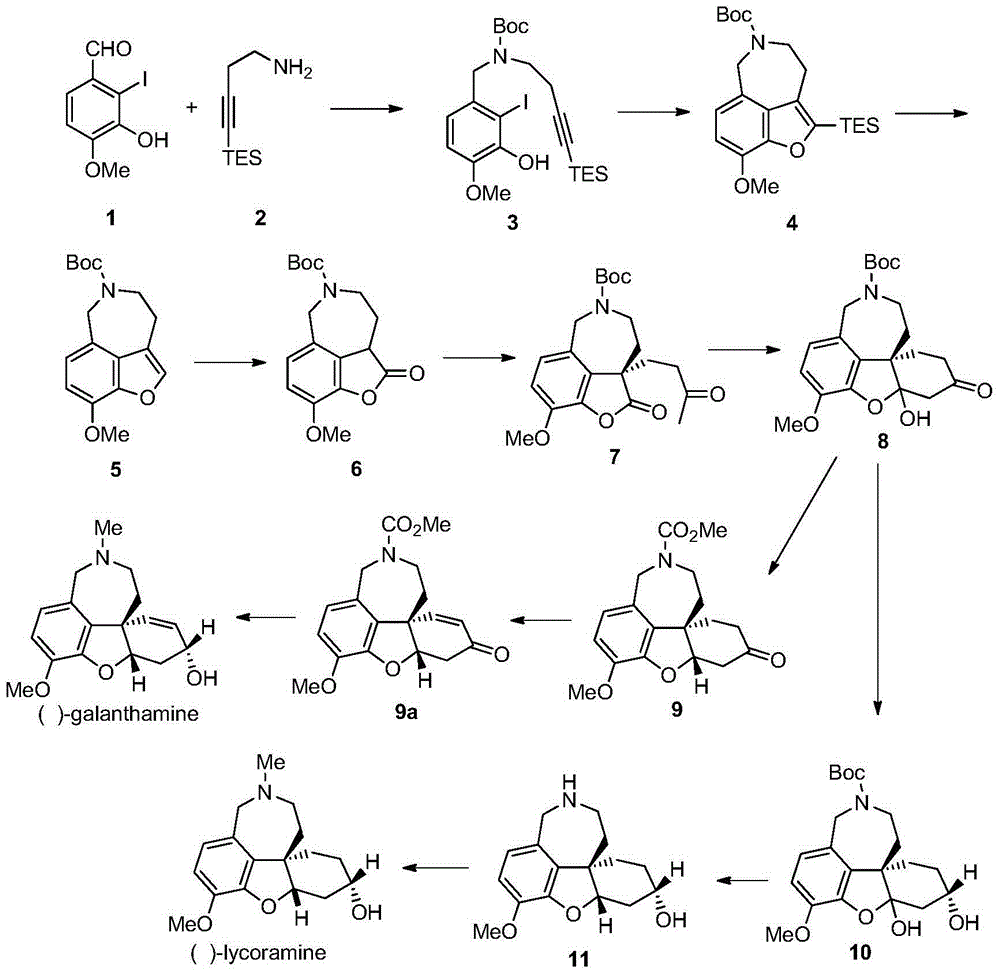
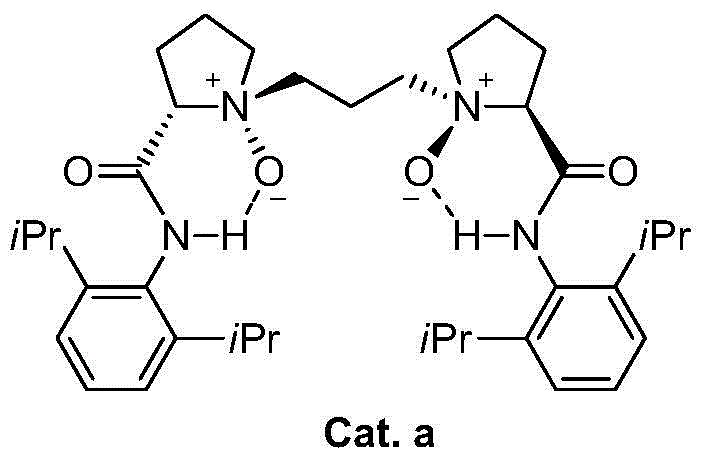
Asymmetric synthesis method of galanthamine and lycoramine
A technology of galantamine and synthesis method, which is applied in the field of synthesis of natural products with complex structures, can solve problems such as low cost, lack of economy, and expensive catalyst, and achieve cost saving, great novelty, and high reaction repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] The following are the specific synthesis process and structural characterization data of the compounds of the present invention.
[0039] Preparation of compound 3
[0040] Compounds 1 (2.51g, 9.0mmol) and 2 (1.64g, 9.0mmol) were dissolved in 25mL of methanol, and at -10°C, NaBH was added 4 (692mg, 18.2mmol, 2.02equiv), stirred at room temperature for 1h. Add triethylamine (1.5mL, 10.8mmol, 1.20equiv) and (Boc) to the reaction solution 2 O (1.96g, 9.1mmol, 1.01equiv), stirring was continued at room temperature for 2h. The reaction solution was concentrated under reduced pressure, then 30 mL of water was added, extracted with ethyl acetate (100 mL×3), and the organic phases were combined with anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, compound 3 (4.9 g) was isolated by column chromatography (petroleum ether / ethyl acetate, 10:1).
[0041] The yield of this step is 81%, and the relevant analysis data are as follows
[0042] 1 H NMR (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com