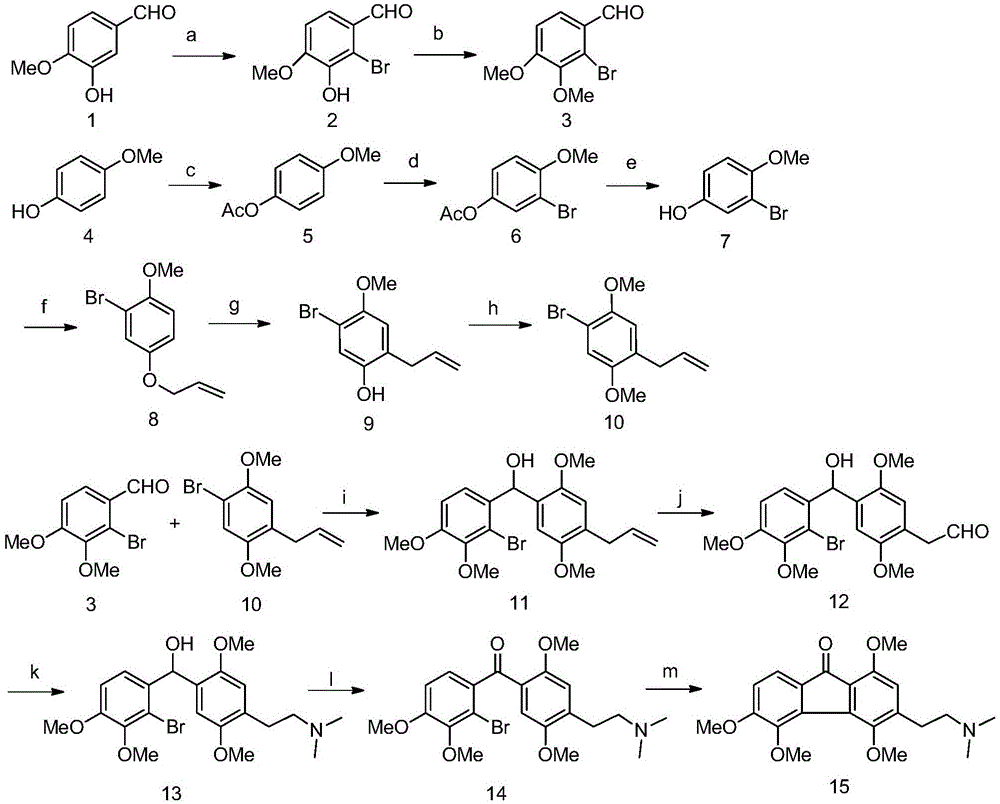
Synthetic method for methyl caulophine
A technology of methyl rhodochrous and dimethylamino, which is applied in the field of synthesizing methyl rhodochrine, can solve the problems that the research on total synthesis of rhodochrine is still blank, and the natural sources of rhodokinine are limited, etc., and achieves good results. Application prospect and research value, cheap and easy availability of reagents, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Step Ⅰ: Compound 2: Synthesis of 2-bromo-3-hydroxy-4-methoxybenzaldehyde
[0040] Weigh 10.00g (65.73mmol) of isovanillin, dissolve it in a 500ml round bottom flask with 200ml glacial acetic acid, add 10.78g (131.45mmol) of sodium acetate, 0.37g (6.57mmol) of iron powder; then take 3.4ml of liquid bromine (65.73mmol) was added to a constant-pressure low-liquid funnel filled with 20ml of glacial acetic acid, and slowly added dropwise to a round-bottomed flask under ice-bath stirring conditions. Add 500ml of ice water, stir for a few minutes, let stand for 10 minutes, filter with suction, wash with ice water until there is no taste of glacial acetic acid, dry and weigh the filter cake to obtain 13.40g of white solid, which is 2-bromo-3-hydroxy-4-methoxy phenylbenzaldehyde (compound 2), yield 88.74%; m.p.206-207°C.
[0041] Step Ⅱ: Compound 3: Synthesis of 2-bromo-3,4-dimethoxybenzaldehyde
[0042] Weigh 10.00g (43.28mmol) of compound 2, dissolve it in a 500ml round-bott...
Embodiment 2
[0065] Step Ⅰ: Compound 2: Synthesis of 2-bromo-3-hydroxy-4-methoxybenzaldehyde
[0066] Weigh 10.00g (65.73mmol) of isovanillin, dissolve it in a 500ml round bottom flask with 200ml glacial acetic acid, add 10.78g (131.45mmol) of sodium acetate, 0.37g (6.57mmol) of iron powder; then take 3.4ml of liquid bromine (65.73mmol) was added to a constant-pressure low-liquid funnel filled with 20ml of glacial acetic acid, and slowly added dropwise to a round-bottomed flask under ice-bath stirring conditions. Add 500ml of ice water, stir for a few minutes, let stand for 10 minutes, filter with suction, wash with ice water until there is no taste of glacial acetic acid, dry and weigh the filter cake to obtain a white solid, which is 2-bromo-3-hydroxy-4-methoxybenzene Formaldehyde (Compound 2); m.p. 206-207°C.
[0067] Step Ⅱ: Compound 3: Synthesis of 2-bromo-3,4-dimethoxybenzaldehyde
[0068] Weigh 10.00g (43.28mmol) of compound 2, dissolve it in a 500ml round bottom flask with dry ac...
Embodiment 3
[0091] Step Ⅰ: Compound 2: Synthesis of 2-bromo-3-hydroxy-4-methoxybenzaldehyde
[0092] Weigh 10.00g (65.73mmol) of isovanillin, dissolve it in a 500ml round bottom flask with 200ml glacial acetic acid, add 10.78g (131.45mmol) of sodium acetate, 0.37g (6.57mmol) of iron powder; then take 3.4ml of liquid bromine (65.73mmol) was added to a constant-pressure low-liquid funnel filled with 20ml of glacial acetic acid, and slowly added dropwise to a round-bottomed flask under ice-bath stirring conditions. Add 500ml of ice water, stir for a few minutes, let stand for 10 minutes, filter with suction, wash with ice water until there is no taste of glacial acetic acid, dry and weigh the filter cake to obtain a white solid, which is 2-bromo-3-hydroxy-4-methoxybenzene Formaldehyde (Compound 2); m.p. 206-207°C.
[0093] Step Ⅱ: Compound 3: Synthesis of 2-bromo-3,4-dimethoxybenzaldehyde
[0094] Weigh 10.00g (43.28mmol) of compound 2, dissolve it in a 500ml round bottom flask with dry ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com