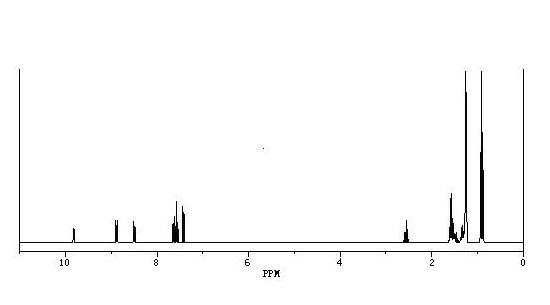
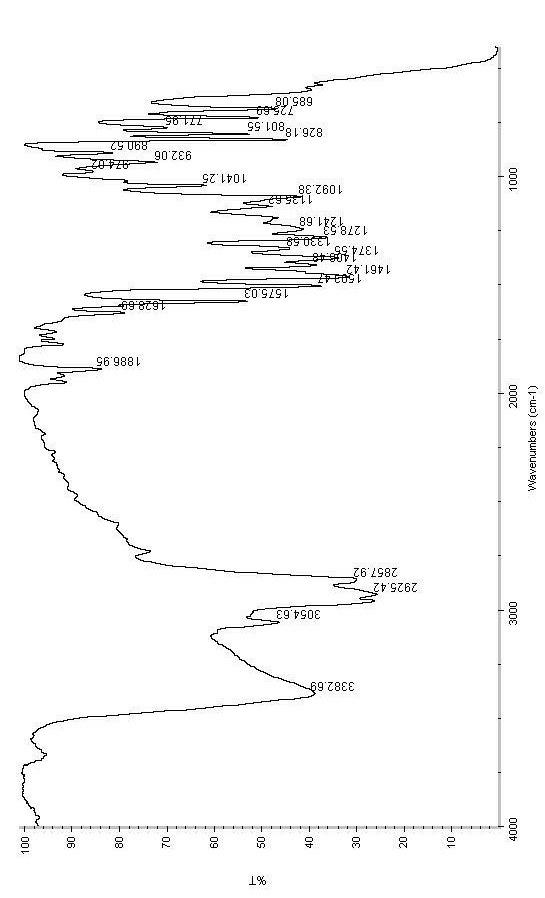
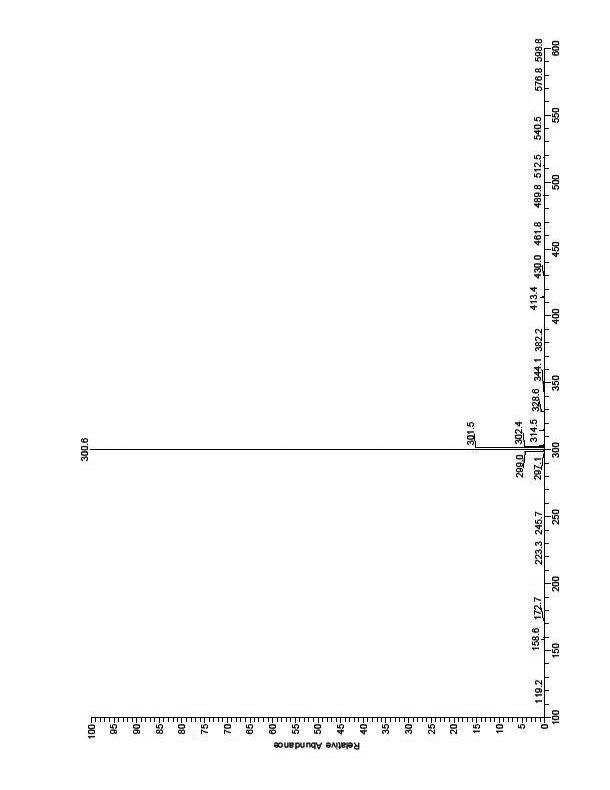
Synthetic method of 7-(4-ethyl-1-methyl octyl)-8-hydroxyquinoline
A technology of hydroxyquinoline and methyl octyl, which is applied in the field of synthesis of 7--8-hydroxyquinoline, can solve the problems of lower product yield, low single-pass conversion rate, condensation by-products, etc., and achieve good quality and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, a kind of synthetic method of 7-(4-ethyl-1-methyloctyl)-8-hydroxyquinoline, its steps are as follows:
[0038] (1) In a solvent, under alkali catalysis, 8-hydroxyquinoline and halogenated alkenes undergo a Williamson synthesis reaction to generate 8-[4-(5-ethyl-2-nonene)]oxyquinoline, and the reaction The temperature is 30°C, phenyl allyl ether undergoes Claisen rearrangement at this reaction temperature, and phenyl allyl ether undergoes intramolecular rearrangement to generate o-allyl 8-hydroxyquinoline, that is, to generate 7-alkenyl -8-hydroxyquinoline, which also generates 7-(4-ethyl-1-methyloctenyl)-8-hydroxyquinoline; the reaction time is 0.5 hours;
[0039] The molar ratio of the raw material halogenated olefin, 8-hydroxyquinoline and solvent is 1:0.5:2;
[0040]The halogenated olefin is 3-chloro-5-ethyl-2-nonene;
[0041] The solvent is N,N-dimethylformamide;
[0042] The catalyst base is selected from sodium hydroxide, potassium hydroxide, pota...
Embodiment 2
[0044] Embodiment 2, a kind of synthetic method of 7-(4-ethyl-1-methyloctyl)-8-hydroxyquinoline, its steps are as follows:
[0045] (1) In a solvent, under alkali catalysis, 8-hydroxyquinoline and halogenated alkenes undergo a Williamson synthesis reaction to generate 8-[4-(5-ethyl-2-nonene)]oxyquinoline, and the reaction The temperature is 180°C, phenyl allyl ether undergoes Claisen rearrangement at this reaction temperature, and phenyl allyl ether undergoes intramolecular rearrangement to generate o-allyl 8-hydroxyquinoline, that is, to generate 7-alkenyl -8-hydroxyquinoline, which also generates 7-(4-ethyl-1-methyloctenyl)-8-hydroxyquinoline; the reaction time is 12 hours;
[0046] The molar ratio of raw material halogenated olefin, 8-hydroxyquinoline and solvent is 1: 6: 12;
[0047] The halogenated olefin is 3-bromo-5-ethyl-2-nonene or 3-iodo-5-ethyl-2-nonene;
[0048] Described solvent is methanol or benzene;
[0049] The catalyst base is made by mixing an inorganic b...
Embodiment 3
[0051] Embodiment 3, a kind of synthetic method of 7-(4-ethyl-1-methyloctyl)-8-hydroxyquinoline, its steps are as follows:
[0052] (1) In a solvent, under alkali catalysis, 8-hydroxyquinoline and halogenated alkenes undergo a Williamson synthesis reaction to generate 8-[4-(5-ethyl-2-nonene)]oxyquinoline, and the reaction The temperature is 100°C, phenyl allyl ether undergoes Claisen rearrangement at this reaction temperature, and phenyl allyl ether undergoes intramolecular rearrangement to generate o-allyl 8-hydroxyquinoline, that is, to generate 7-alkenyl -8-hydroxyquinoline, which also generates 7-(4-ethyl-1-methyloctenyl)-8-hydroxyquinoline; the reaction time is 6 hours;
[0053] The molar ratio of raw material halogenated olefin, 8-hydroxyquinoline and solvent is 1:3:7;
[0054] The halogenated olefin is 3-iodo-5-ethyl-2-nonene;
[0055] Described solvent is toluene or cyclohexane;
[0056] The catalyst base is made by mixing an inorganic base with a low-carbon monohyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com