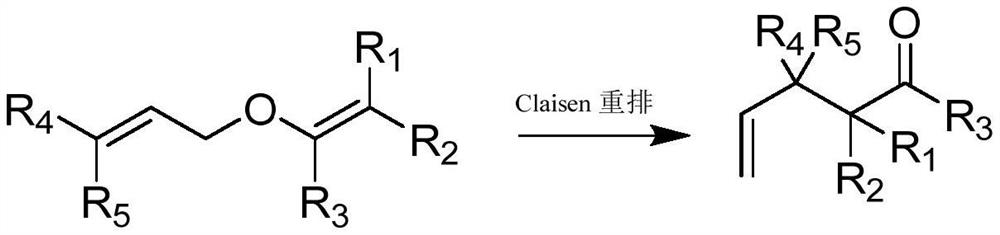
Method for catalyzing Claisen rearrangement at lower temperature
A rearrangement and catalyst technology, applied in the field of catalysis, can solve problems such as high pressure, high energy consumption, and low product quality, and achieve the effects of reducing rearrangement reaction temperature, reducing production energy consumption, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
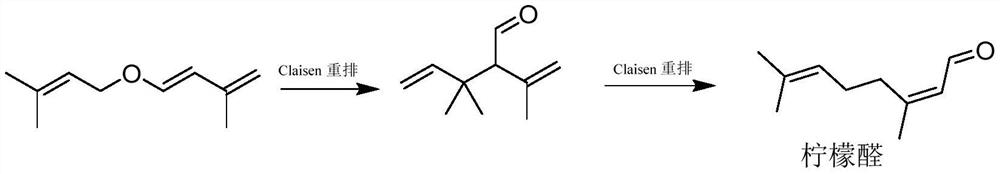
[0030] With cis / trans-isopentenyl-3-methylbutadiene ether 152.24g (1.0mol) and the catalyst shown in the following formula:
[0031]
[0032] That is, 2-ethoxy-1,3-dimethyl-2,3-dihydro-1H-benzo[d][1,3,2]diazaphosphole105.1mg (0.5mmol) (relative to raw material cis / trans-isopentenyl -3-Methylbutadiene ether (0.05mol%) was added into the tank reactor, and then the reactor was replaced with nitrogen three times. After the replacement, the stirring was started and the temperature was raised to about 80°C. , the remaining raw materials are detected by GC <3%; open three sets of decompression pumps, and slowly increase the temperature to 110°C, at this time, the pressure in the kettle is about 400Pa, keep the temperature in the kettle at 110-120°C and distill under reduced pressure until no liquid drops flow out . Collect the distillate to get 3,3-dimethyl-2-isopropenyl-4-pentene-1-aldehyde (isomer) and 3,7-dimethyl-2,6-octadienal (lemon Aldehyde) mixture 139.9 g, yield 90%, GC...
Embodiment 2
[0036] With 1522.4g toluene, cis / trans-isopentenyl-3-methylbutadiene ether 152.24g (1.0mol), the catalyst shown in the following formula:
[0037]
[0038] That is, 2,5,6-trimethoxy-1,3-dimethyl-2,3-dihydro-1H-benzo[d][1,3,2]diazaphosphole1281.25mg (5mmol) (relative to raw material cis / trans-iso Pentenyl-3-methylbutadiene ether (0.5mol%) was added into the tank reactor, and then the reactor was replaced with nitrogen three times. After the replacement, the stirring was started and the temperature was raised to about 120°C. After reacting for 60 minutes, no residual raw materials were detected by GC; first, a group of decompression pumps were turned on, and the pressure in the kettle was reduced to 1500pa to distill out the low boiling point solvent p-xylene, and the temperature was kept at 50-80°C; the pressure was reduced until there was no liquid drop After flowing out, turn on three sets of decompression pumps, and slowly raise the temperature to 110°C at the same time. ...
Embodiment 3
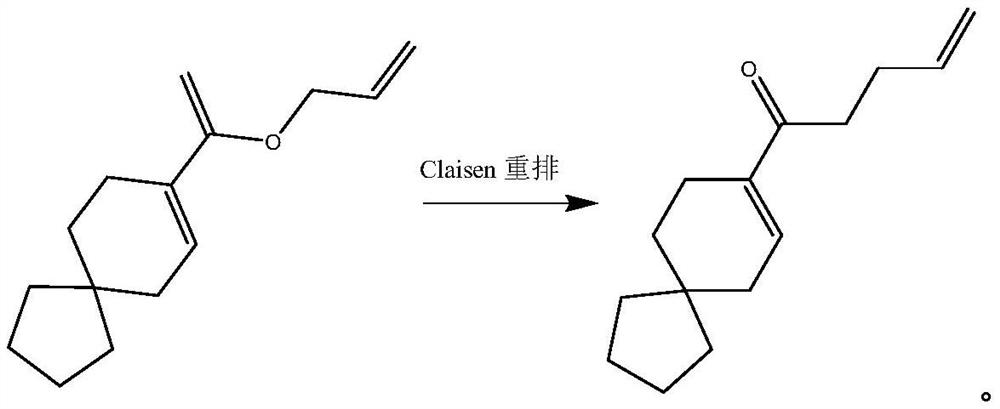
[0042] With 1091g xylene, 8-(1-(allyloxy)vinyl)spiro[4.5]dec-7-ene 218.34g (1mol), the catalyst shown in the following formula:
[0043]
[0044]That is, 2,5,6-triethoxy-1,3-dimethyl-2,3-dihydro-1H-benzo[d][1,3,2]diazaphosphole298.32mg (1mmol), was added to the tank reactor followed by The reaction kettle was replaced with nitrogen three times. After the replacement, the stirring was started, and the temperature was raised to about 70°C. After 5 hours of reaction, no residue of raw materials was detected by GC. First turn on a group of decompression pumps, reduce the pressure in the kettle to 1500pa to distill out the low boiling point solvent xylene, and depressurize until no liquid drops flow out; turn on three sets of decompression pumps, and slowly increase the temperature to 130°C at the same time. The pressure in the kettle is about 300Pa, and the temperature in the kettle is kept at 130°C for vacuum distillation until no liquid drops flow out. The distillate was col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com