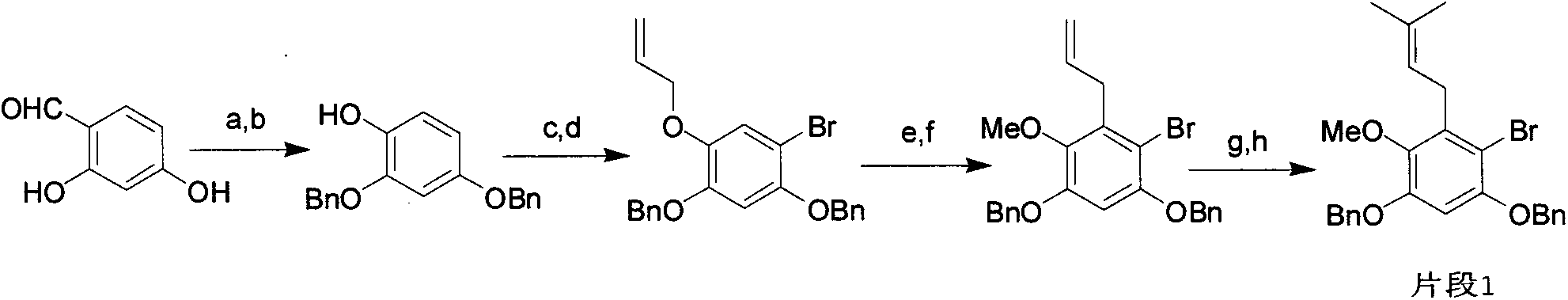
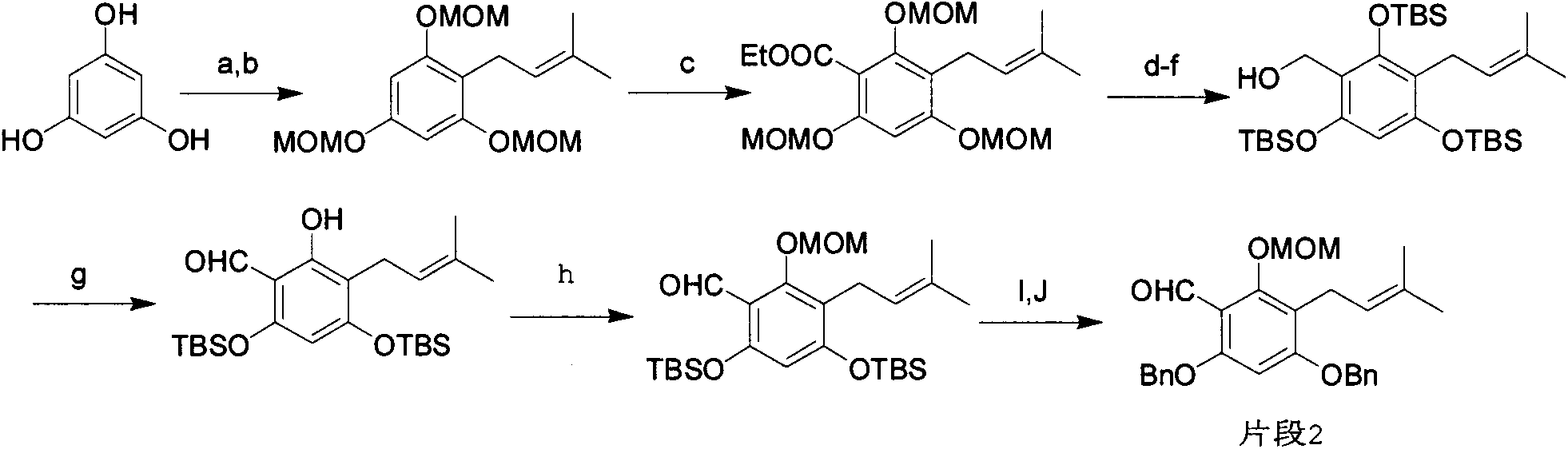
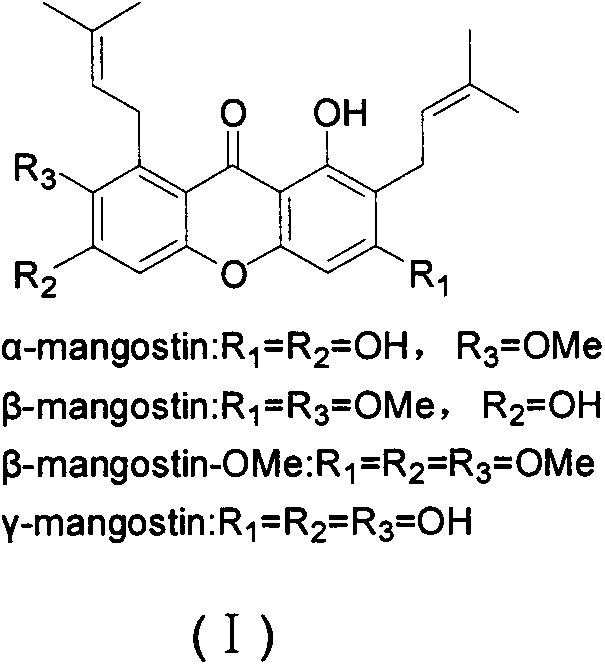Novel synthesis method of mangostin
A mangostin, a new synthesis technology, applied in the direction of organic chemistry, etc., can solve the problems of long cycle, cumbersome steps, low yield and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of 1,7-(1,1-dimethyl-allyloxy)-3,6-dimethoxy-9H-xanthone (IV):
[0047] Dissolve 330 mg (1.15 mmol) of 1,7-dihydroxy-3,6-dimethoxy-9H-xanthone (III) in 40 ml of acetone, add 256 mg (4.58 mmol) of potassium hydroxide and 10 mg of potassium iodide in sequence, 3-Chloro-3-methyl-1-butene (II), refluxed for 12 hours, and after suction filtration, column chromatography (petroleum ether: ethyl acetate = 4: 1) gave yellow powdery solid 1,7-(1 , 1-dimethyl-allyloxy)-3,6-dimethoxy-9H-xanthone (IV) 414 mg, yield 85.2%.
[0048] 1 H-NMR (300MHz, CDCl 3 ): δ1.68 (9H, s, -CH 3 ×3), δ1.76 (3H, s, -CH 3 ), δ3.93, 3.97 (3H each, s, -OCH 3 ×2), δ4.64(4H, d, =CH 2 ×2), δ5.54, 6.64 (1H each, t, -CH=×2), δ6.32, 6.44, 6.79, 7.67 (1H, s, C 2 , C 4 , C 5 , C 8 -H), δ13.78 (1H, s, C 1 -OH);
[0049] ESI-MS (m / z): 437[M+Na] + .
Embodiment 2
[0051] Preparation of 1,7-dihydroxy-3,6-dimethoxy-2,8-prenyl-9H-xanthone (V):
[0052] Dissolve 424 mg (1 mmol) of 1,7-(1,1-dimethyl-allyloxy)-3,6-dimethoxy-9H-xanthone (IV) in 15 ml of N,N-dimethyl To aniline, add anhydrous aluminum chloride 10mg, N 2 Heat up under protection, reflux for 4 hours, cool to room temperature, add 15% HCl and stir for 20 min, extract with ethyl acetate, combine organic phases, wash once with 10% HCl, and dry over anhydrous sodium sulfate. After column chromatography (petroleum ether: ethyl acetate = 8:1), yellow solid 1,7-dihydroxy-3,6-dimethoxy-2,8-diprenyl-9H-xanthone was obtained (V) 276 mg, yield 65.1%.
[0053] 1 H NMR (300MHz, DMSO-D6): δ1.64 (6H, s, -CH 3 ×2), δ1.79 (3H, s, -CH 3 ), δ1.89 (3H, s, -CH 3 ), 3.34 (2H, d, J=9.12Hz, -CH 2 -), δ3.79~4.14 (6H, t, -OCH 3 ×2), δ4.12 (2H, m, -CH 2 -), δ5.18(2H, m, -CH=×2), δ6.57(1H, s, Ar-CH), δ6.73(1H, s, Ar-CH), δ9.12(1H, s, Ar-OH), δ13.4 (1H, s, Ar-OH);
[0054] EI-MS (m / z): 424[M] + ....
Embodiment 3
[0056] Preparation of 1-hydroxy-3,6,7-trimethoxy-2,8-diprenyl-9H-xanthone (β-mangostin-OMe) (VI):
[0057] Dissolve 300 mg (0.707 mmol) of 1,7-dihydroxy-3,6-dimethoxy-2,8-diprenyl-9H-xanthone in 30 ml of acetone, and add 195 mg of potassium carbonate (1.41 mmol), dimethyl sulfate 161mg (1.272mmol), reflux reaction for four hours, evaporate the solvent under reduced pressure, add 10% NaOH solution and reflux for 1 hour, cool to room temperature, adjust pH to acidity, extract three times with ethyl acetate, combine organic phase, dried over anhydrous sodium sulfate, and separated by column chromatography (petroleum ether: ethyl acetate = 8:1), to obtain 1-hydroxyl-3,6,7-trimethoxy-2,8-diisoamyl as a yellow oil Alkenyl-9H-xanthone (VI) 287 mg, yield 92.6%.
[0058] mp: 116-118°C;
[0059] 1 H NMR (300MHz, DMSO-D6): δ1.72 (6H, s, -CH 3 ×2), δ1.77 (6H, s, -CH 3 ×2), δ3.28(2H, d, J=7.2Hz, -CH 2 -), δ3.68 (3H, s-OCH 3 ), δ3.90 (3H, s-OCH 3 ), δ3.96 (3H, s-OCH 3 ), δ4.02 (2H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com