Synthesis method of isolicoflavonol
A technology of isolicorice flavonol and synthesis method, applied in organic chemistry, bulk chemical production, etc., can solve the problems of high cost, low output, long preparation process, etc., and achieve low production cost, high product yield, and simple raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] As shown in the above formula (II), the synthetic method according to the present invention specifically includes:
[0113] Step S1, the 2,4-O-protecting group R 1 -6-hydroxyl-acetophenone (compound shown in structural formula 3) and 4-O-protecting group R 2 -benzaldehyde (compound shown in structural formula 5) is condensed to generate 2', 4'-O-protecting group R 1 -6'-Hydroxy-4-O-protecting group R 2 - Chalcone (compound represented by structural formula 6).
[0114] Wherein, the protecting group R 1 , R 2 independently selected from -CH 3 ,-CH 2 OCH 3 , the group formed by benzyl, allyl, acetyl, isopentenyl, 1,1-dimethyl-propynyl. That is, the protecting group R 1 , R 2 They can be the same or different, and there is no interdependence between them, and they can be arbitrarily selected from the above-mentioned groups.
[0115] Specifically, the 2,4-O-protecting group R 1 -6-hydroxyl-acetophenone can be prepared through the following steps:
[0116] In st...
example 1
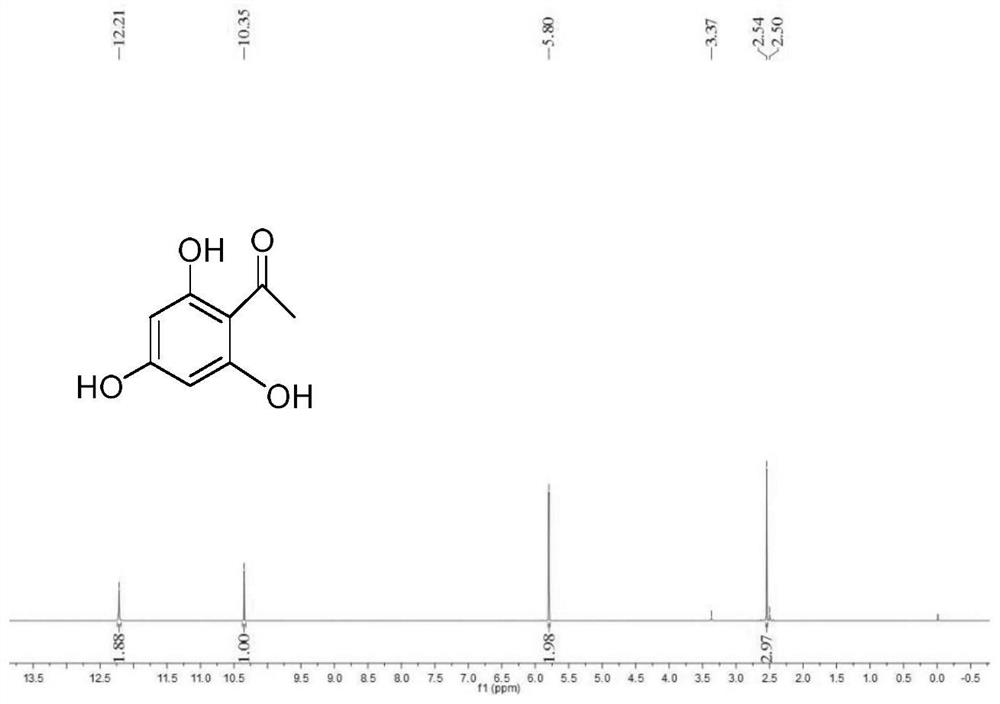
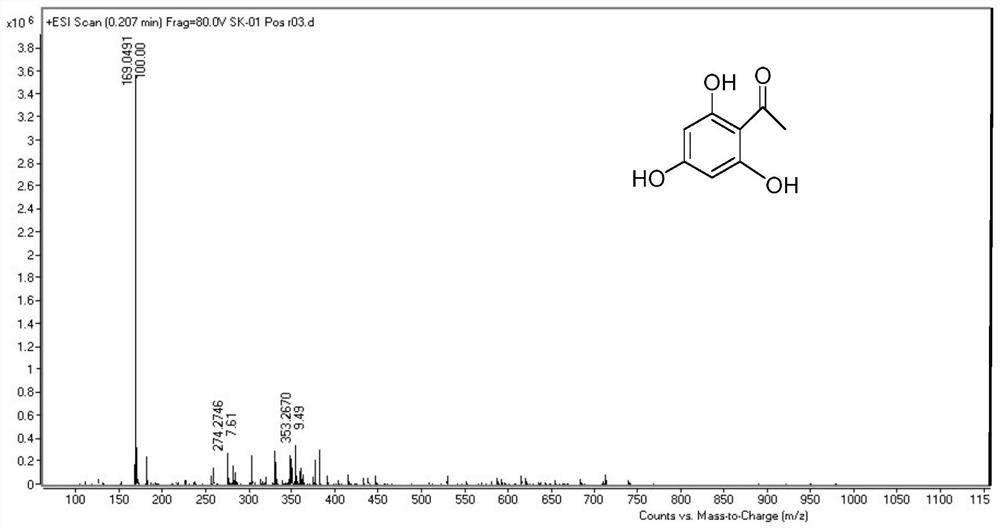
[0141] Example 1 Synthesis of 2,4,6-trihydroxyacetophenone (compound shown in structural formula 2)
[0142] Under cooling in an ice-salt bath, add phloroglucinol 1 (10.0 g, 0.080 mol), anhydrous acetonitrile (9.7 ml, 0.185 mol), anhydrous ZnCl 2 (2.81g, 0.021mol) and 42ml of anhydrous diethyl ether, under stirring, dry HCl gas was passed through for 6h, and a large amount of light yellow solid was formed.
[0143] Stop the reaction, put the reaction solution in the refrigerator and let it stand for 3d. Access to N 2The residual HCl gas was removed, filtered, and the filter cake was washed twice with 20 ml of anhydrous ether. The filter cake and 2.5 g of activated carbon were added to 500 ml of deionized water, refluxed for 2.5 h, filtered while hot, and recrystallized to obtain pale yellow needle crystals. The solid was vacuum-dried at 120°C for 4 h to obtain 10.76 g of orange-red crystals (yield: 80.7%) .
[0144] m.p.215.4-217.7℃.IRνmax(KBr / cm-1):3291(OH),1626(C=O),1590...
example 2
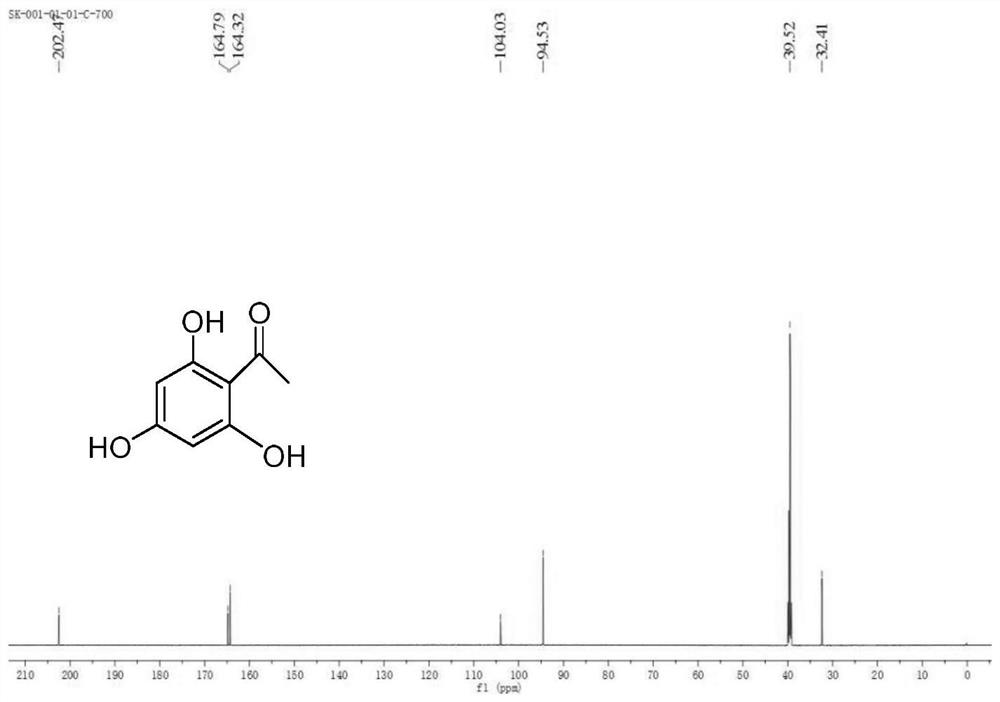
[0149] Example 2 Synthesis of 2,4-dimethoxy-6-hydroxyl-acetophenone (one of the compounds shown in structural formula 3, denoted as 3a)
[0150] Compound 2 (10.0g, 0.059mol) was dissolved in 100ml of acetone, anhydrous potassium carbonate (24.58g, 0.178mol) was added, under stirring, dimethyl sulfate (13ml, 0.137mol) was added dropwise, heated to reflux for 1.5h, Cool to room temperature, remove solid potassium carbonate by filtration to obtain an orange-yellow acetone solution, and rotary evaporate to obtain 11.24 g of a yellow crude product. The crude product is recrystallized with 50 ml of ethanol-water to obtain a white solid, and then vacuum-dried at 45 ° C for 12 hours to obtain an orange-red solid 9.25 g (85.7% yield).
[0151] m.p.74.5-77.0°C, 1 H NMR (400MHz, DMSO) δ13.80(s, 1H), 6.09(d, J=2.3Hz, 1H), 6.06(d, J=2.3Hz, 1H), 3.85(s, 3H), 3.80(s ,3H),2.53(s,3H).
[0152] 13 C NMR (101MHz, DMSO) δ202.76, 166.23, 165.94, 162.77, 105.44, 93.61, 90.76, 55.98, 55.65, 32.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com