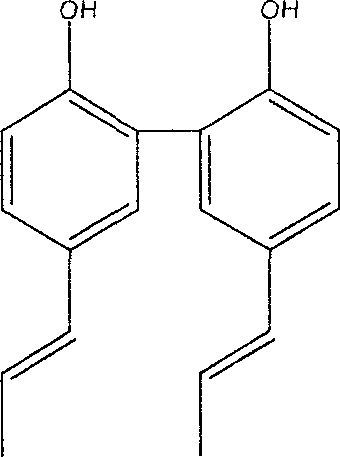
Preparation method of magnolia bark phenol derivative
A technology of magnolol and its derivatives, which is applied in the field of preparation of magnolol derivatives, can solve the problems such as synthesis research rarely reported, and achieve the effects of enhanced oil solubility, good symmetry, and strong antioxidant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
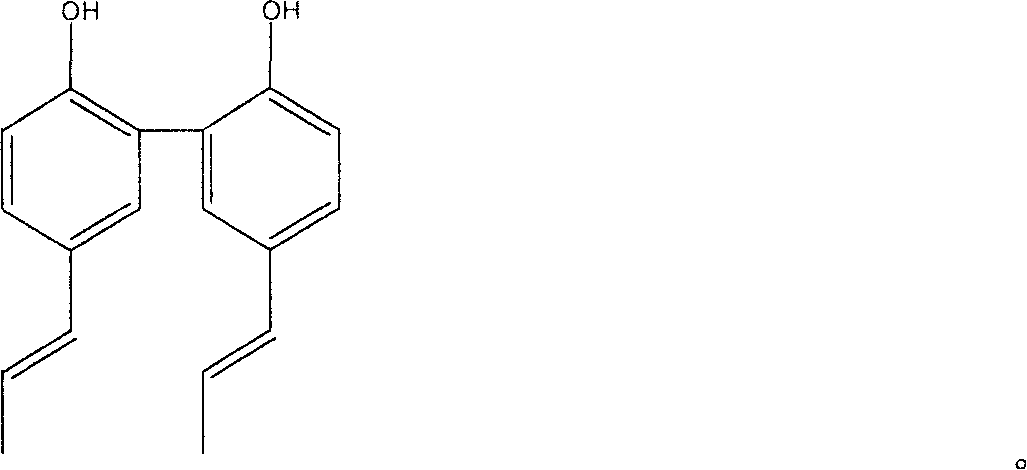
[0026] 5,5'-dipropenyl biphenol is generated by isomerization reaction with magnolol.
[0027] First, add 0.15 mol of potassium hydroxide and 0.15 mol of glycerin into a 100ml two-necked flask. One of the two-necked flasks is connected to a spherical condenser tube, and the other is connected to a nitrogen tube. The flask is placed on a constant temperature magnetic stirrer, and the heating switch and stirring switch are turned on. Heating and stirring are carried out so that all potassium hydroxide is dissolved in glycerin, and nitrogen protection is introduced at the same time. Continue to heat up to 140°C, add 0.01mol of magnolol ground into powder, then raise the oil bath temperature to 190°C for reaction, the reaction time is 2 to 3 hours; after the reaction, the reaction product is cooled to room temperature, and Adjust the pH to about 2-3 with 20% sulfuric acid, add an appropriate amount of distilled water, and stir to make it uniform; extract the above mixture with eth...
Embodiment 2
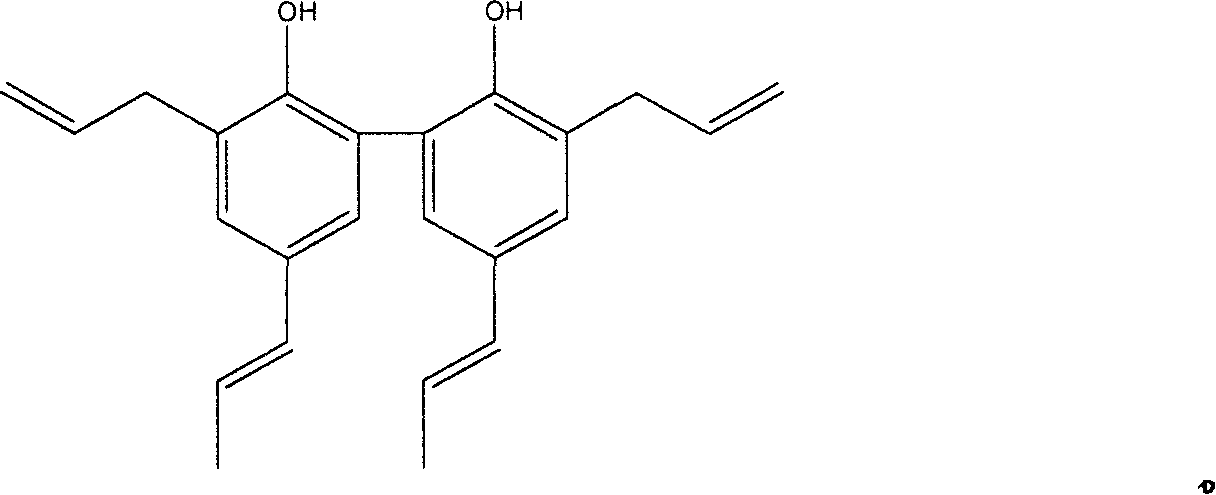
[0029] Preparation of 3,3'-diallyl-5,5'-dipropenyl biphenol, the first step of the reaction is the Williamson reaction of 5,5'-dipropenyl biphenol and allyl bromide Synthesis of allyl ether; in the second step, Claisen rearrangement of allyl ether can generate 3,3'-diallyl-5,5;--dipropenyl biphenol.
[0030] Add 30ml of acetone, 0.01mol of magnolol and 0.02mol of anhydrous potassium carbonate into a 100ml single-necked flask, then add 0.026mol of allyl bromide into the flask (ice bath), connect the flask to a spherical condenser, and place the flask under a constant temperature magnetic force On the stirrer, turn on the heating switch and the stirring switch, carry out heating and stirring, and finish the reaction at 65° C. for 6 hours. The product was filtered to remove K 2 CO, concentrated by distillation, and applied to a silica gel column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain a pale yellow oil. Transfer it to a 50mo two-necked flask. One of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com